Clausius Clapeyron Equation
The Clausius Clapeyron Equation Derivation With Applications Clausius–clapeyron equation. the clausius–clapeyron equation [7]: 509 applies to vaporization of liquids where vapor follows ideal gas law using the specific gas constant and liquid volume is neglected as being much smaller than vapor volume v. it is often used to calculate vapor pressure of a liquid. [8]. Learn how to apply the clausius clapeyron equation to estimate the vapor pressure and heat of phase transition of liquids and solids. see examples, derivations and exercises with solutions.

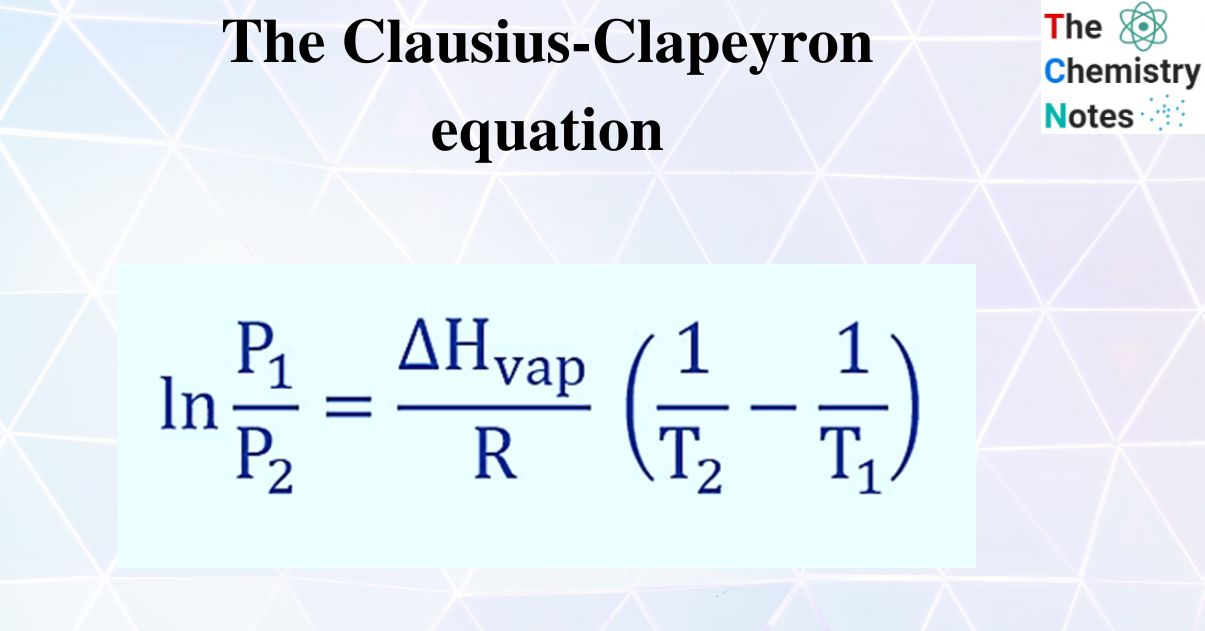
Clausius Clapeyron Equation Learn how the clausius clapeyron equation relates the vapor pressure of a substance to its temperature and phase transitions. find out how to derive, apply, and plot the equation in physical chemistry and related fields. Learn how to use the clausius clapeyron equation to predict the vapor pressure or heat of phase transition of a substance from vapor pressure at two temperatures. see the formula, examples, and applications in physical chemistry, thermodynamics, and meteorology. Equation 23.4.1 is known as the clausius clapeyron equation. we can further work our the integration and find the how the equilibrium vapor pressure changes with temperature: ln(p2 p1) = − Δhvap molar r [1 t2 − 1 t1] thus if we know the molar enthalpy of vaporization we can predict the vapor lines in the diagram. Learn how to use the clausius clapeyron equation to calculate the enthalpy of vaporization and the vapor pressure of a substance. see examples, formulas, units and solutions for different problems.

Comments are closed.