Clapeyron Equation
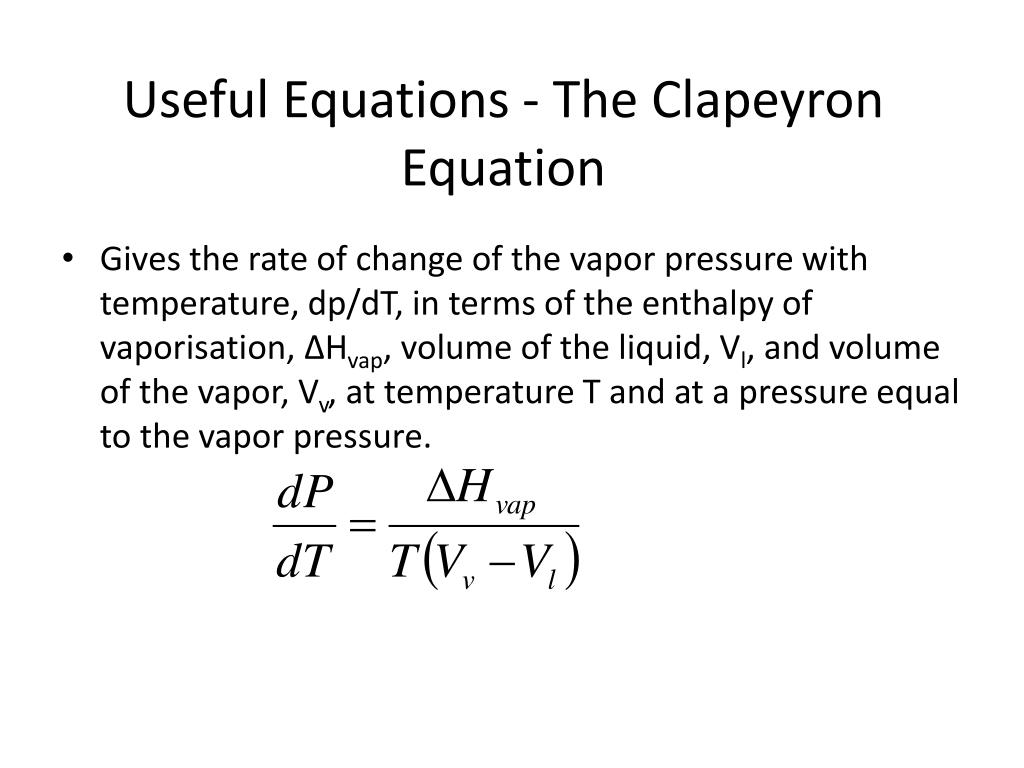
Ppt Useful Equations The Clapeyron Equation Powerpoint Presentation Learn how the clapeyron equation relates the changes in pressure and temperature for a phase change, and how it affects the shape of the phase diagram for water. see an example calculation of the freezing point depression for water under pressure. Clausius–clapeyron relation. the clausius–clapeyron relation, in chemical thermodynamics, specifies the temperature dependence of pressure, most importantly vapor pressure, at a discontinuous phase transition between two phases of matter of a single constituent. it is named after rudolf clausius [1] and benoît paul Émile clapeyron. [2].
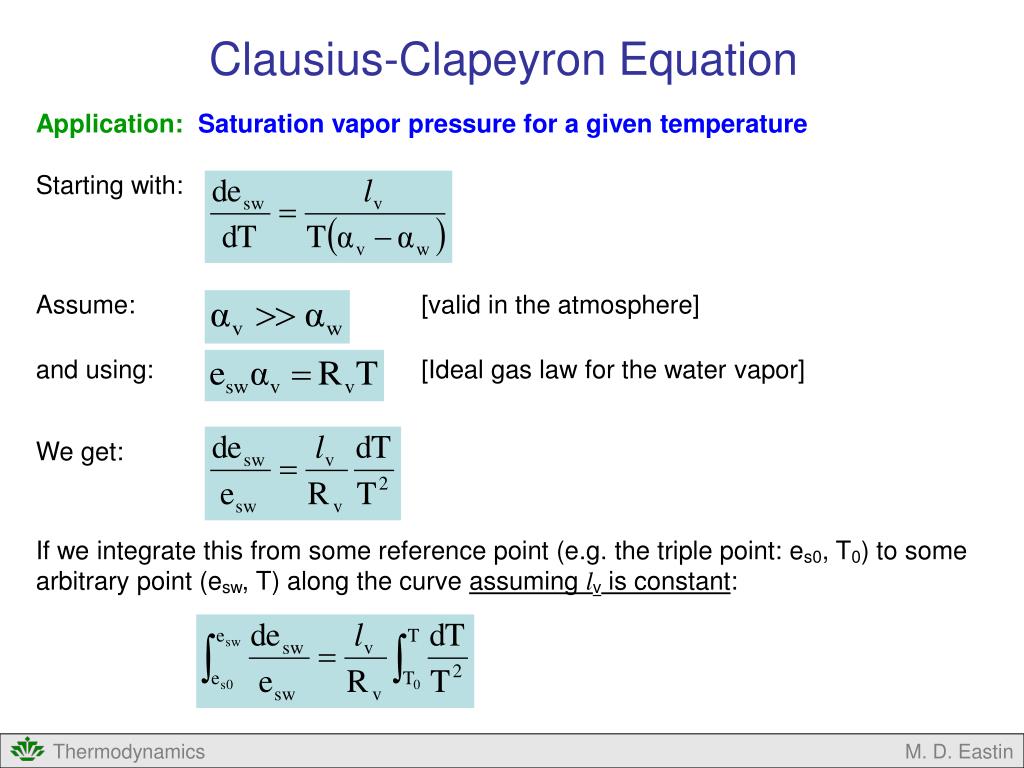
Ppt Clausius Clapeyron Equation Powerpoint Presentation Free Learn how to apply the clausius clapeyron equation to estimate the vapor pressure and heat of phase transition of liquids and solids. see examples, derivations and exercises with solutions. Learn how to use the clausius clapeyron equation to predict the vapor pressure or heat of phase transition of a substance from vapor pressure at two temperatures. see the formula, examples, and applications in physical chemistry, thermodynamics, and meteorology. Learn how the clausius clapeyron equation relates the vapor pressure of a substance to its temperature and phase changes. find out how to derive, apply, and plot the equation in physical chemistry and thermodynamics. Learn how to use the clapeyron equation to relate the pressure volume relationship of a substance to its phase change. see how to derive the equation from the clausius clapeyron relation and how to apply it to calculate the heat of vaporization, the melting point, and the boiling point of a substance.

Clausius Clapeyron Equation Learn how the clausius clapeyron equation relates the vapor pressure of a substance to its temperature and phase changes. find out how to derive, apply, and plot the equation in physical chemistry and thermodynamics. Learn how to use the clapeyron equation to relate the pressure volume relationship of a substance to its phase change. see how to derive the equation from the clausius clapeyron relation and how to apply it to calculate the heat of vaporization, the melting point, and the boiling point of a substance. Learn how the clausius clapeyron equation relates the variation of pressure and temperature at phase changes, such as the boiling point of water. see the derivation, examples and applications of this thermodynamic formula. The clapeyron equation. the clapeyron attempts to answer the question of what the shape of a two phase coexistence line is. in the \(p t\) plane, we see the a function \(p(t)\), which gives us the dependence of \(p\) on \(t\) along a coexistence curve. consider two phases, denoted \(\alpha\) and \(\beta\), in equilibrium with each other.
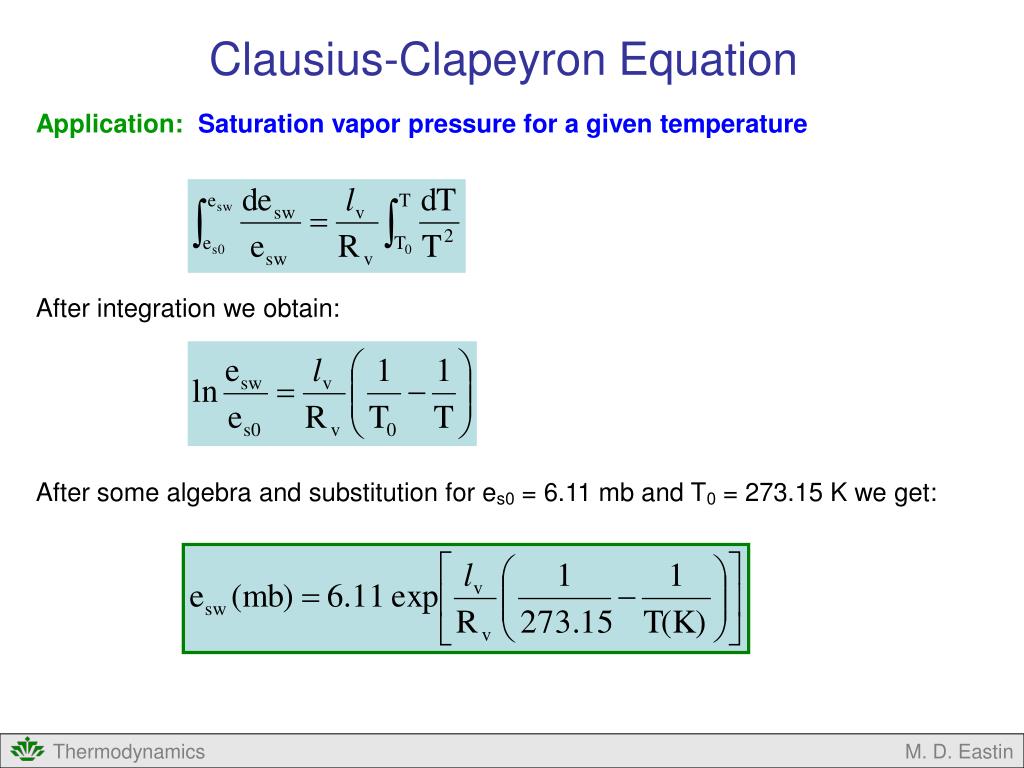
Ppt Clausius Clapeyron Equation Powerpoint Presentation Free Learn how the clausius clapeyron equation relates the variation of pressure and temperature at phase changes, such as the boiling point of water. see the derivation, examples and applications of this thermodynamic formula. The clapeyron equation. the clapeyron attempts to answer the question of what the shape of a two phase coexistence line is. in the \(p t\) plane, we see the a function \(p(t)\), which gives us the dependence of \(p\) on \(t\) along a coexistence curve. consider two phases, denoted \(\alpha\) and \(\beta\), in equilibrium with each other.
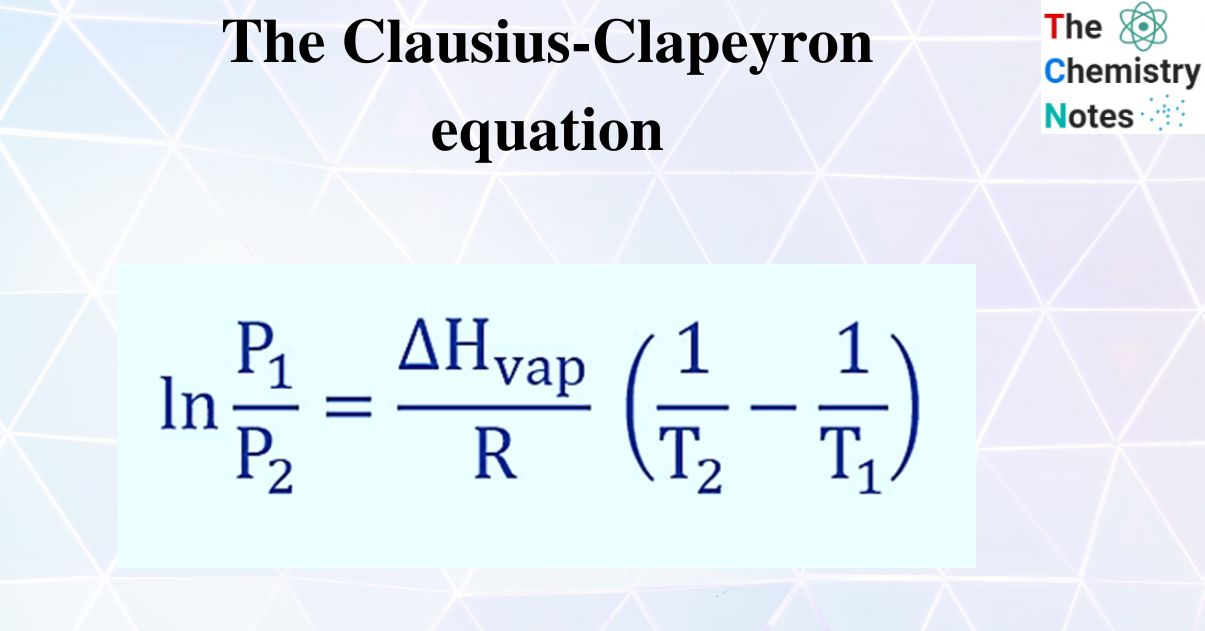
The Clausius Clapeyron Equation Derivation With Applications

Comments are closed.