Chemistry Polyatomic Ions Chart
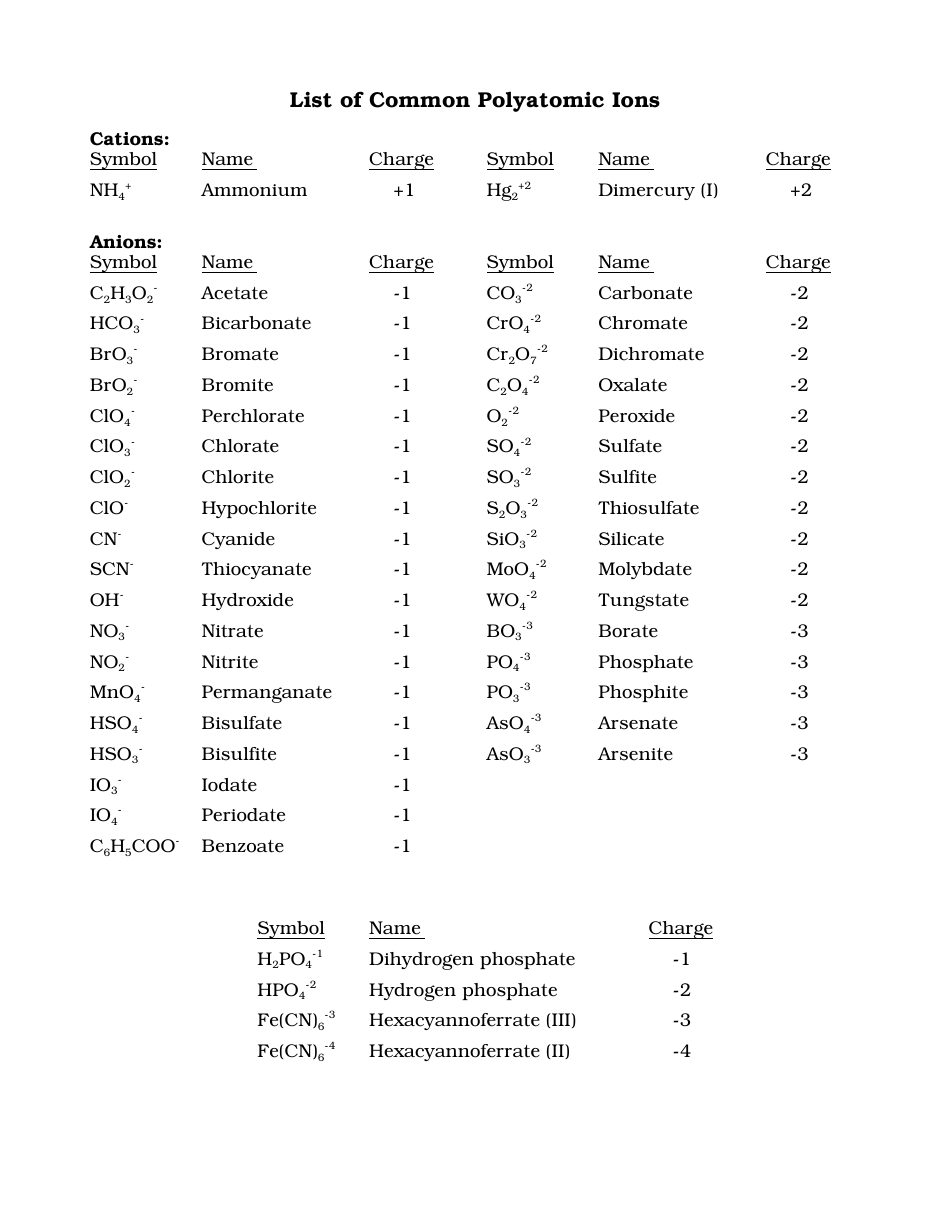
Common Polyatomic Ions Chart Cations Anions Download Printable Pdf A comprehensive list of polyatomic ions and their names, formulas, and charges. also includes some metallic cations and their names and formulas. Table 3.6.1 3.6. 1: common polyatomic ion names and formulas. note that only two polyatomic ions in this table are cations, hydronium ion (h 3 o ) and ammonium ion (nh 4 ), the remaining polyatomic ions are all negatively charged and, therefore, are classified as anions. however, only two of these, the hydroxide ion and the cyanide ion, are.

Names And Formulas Of Polyatomic Ions Common polyatomic ions name(s) formula name(s) formula ammonium nh4 acetate ch3coo c2h3o2 bromate bro3 carbonate co3 2 chlorate clo3 chlorite clo2 chromate cro4 2 cyanide cn dichromate cr2o7 2 hydrogen carbonate bicarbonate hco3 hydrogen sulfate bisulfate hso4 hydrogen phosphate biphosphate hpo4 2 hydroxide oh. Ion name. oxalate chromite chromate dichromate ferricyanide ferrocyanide hydrogen arsenate. hydrogen carbonate. hydrogen phosphite hydrogen phosphate dihydrogen phosphite dihydrogen phosphate hydrogen sulfite hydrogen sulfate hypoiodite iodite ion symbol. Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. table 3.4.1 3.4. 1 lists the ion names and ion formulas of the most common polyatomic ions. for example, no−3 no 3 − is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1− charge. Formula. structure. phosphate. po 43 . phosphite. po 33 . this polyatomic ions list contains many common polyatomic ions grouped by charge. each entry contains the ion's name, molecular formula and chemical structure.
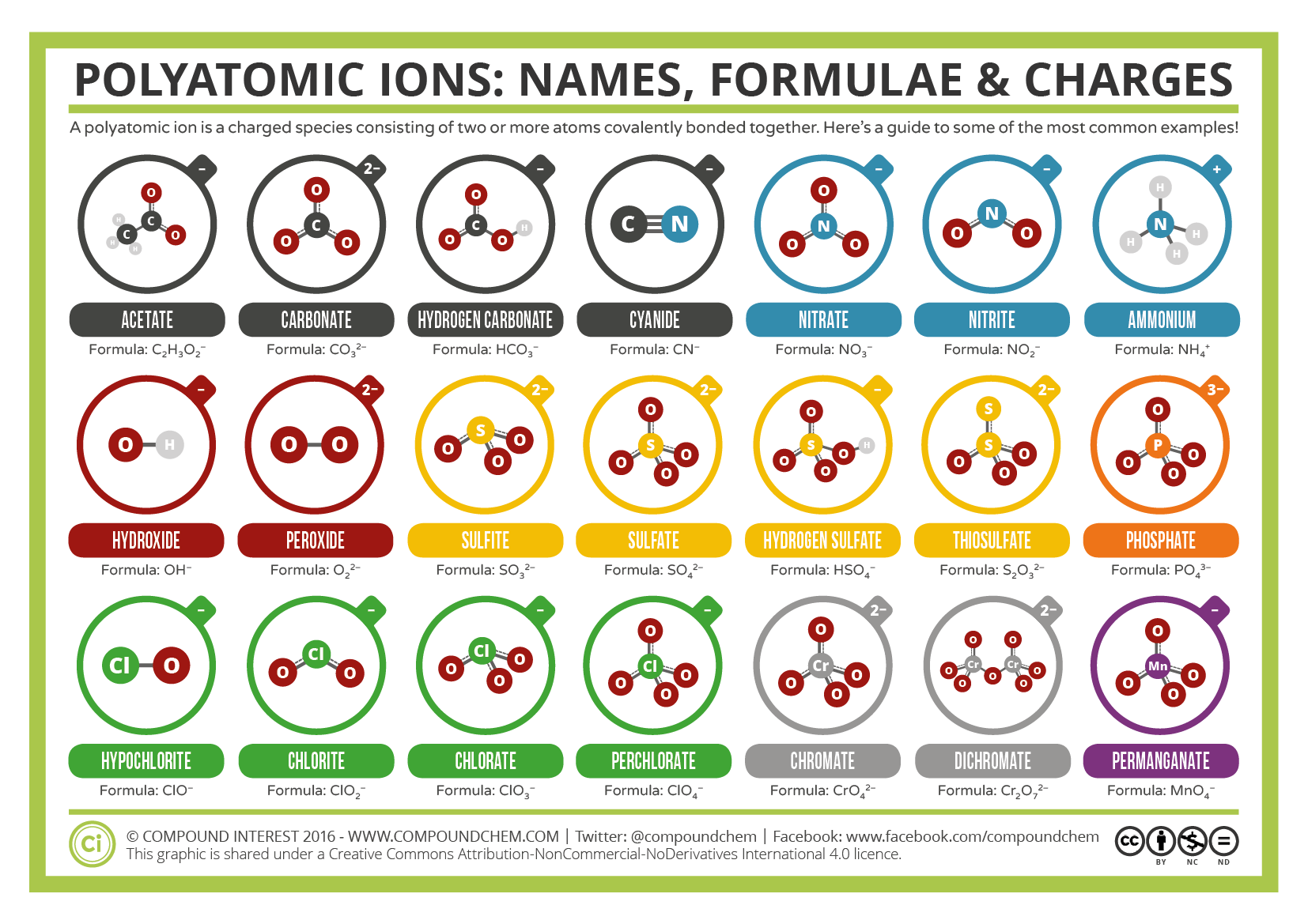
Common Polyatomic Ions Names Formulae And Charges Compound Interest Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. table 3.4.1 3.4. 1 lists the ion names and ion formulas of the most common polyatomic ions. for example, no−3 no 3 − is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1− charge. Formula. structure. phosphate. po 43 . phosphite. po 33 . this polyatomic ions list contains many common polyatomic ions grouped by charge. each entry contains the ion's name, molecular formula and chemical structure. Some polyatomic ions can be changed by change number of oxygens. ate ion ite ion per ite (base) formula ( 1 oxy) formula ( 1 oxy) chlorate clo31 chlorite clo21 perchlorate bromate bro31 bromite bro21 perbromate. nitrate no31 nitrite no21 carbonate co32 carbonite co22 sulfate so42 sulfite so32 phosphate po43 phosphite po33 . Table 6.3.1 6.3. 1: some polyatomic ions. polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. table 6.3.2 6.3. 2 lists the ion names and ion formulas of the most common polyatomic ions. for example, no−3 no 3 − is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1−.
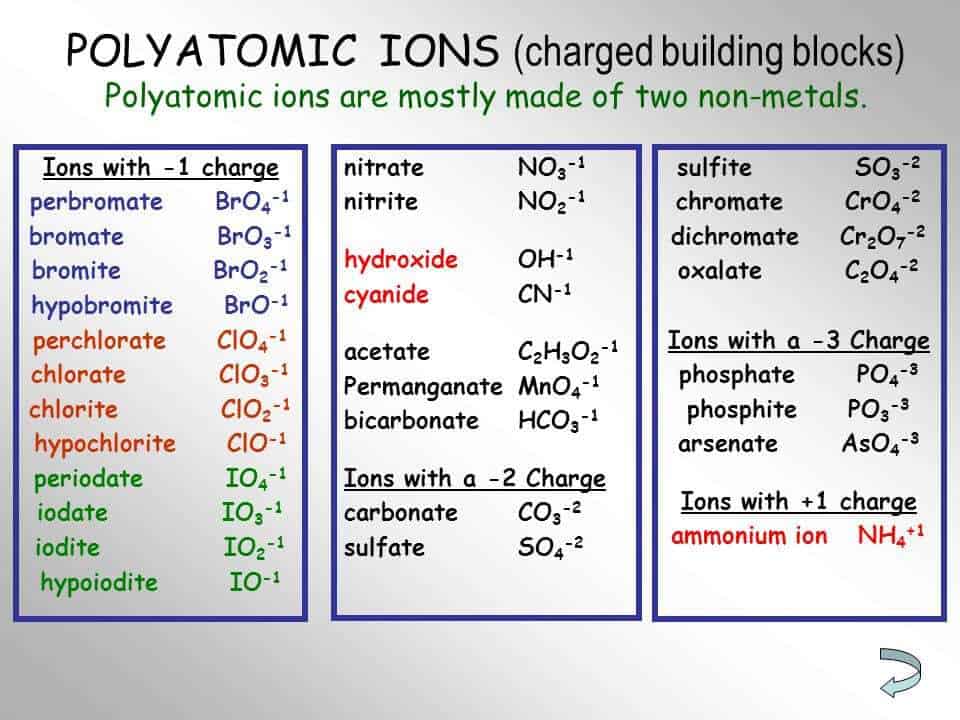
Polyatomic Ion Charts Find Word Templates Some polyatomic ions can be changed by change number of oxygens. ate ion ite ion per ite (base) formula ( 1 oxy) formula ( 1 oxy) chlorate clo31 chlorite clo21 perchlorate bromate bro31 bromite bro21 perbromate. nitrate no31 nitrite no21 carbonate co32 carbonite co22 sulfate so42 sulfite so32 phosphate po43 phosphite po33 . Table 6.3.1 6.3. 1: some polyatomic ions. polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. table 6.3.2 6.3. 2 lists the ion names and ion formulas of the most common polyatomic ions. for example, no−3 no 3 − is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1−.

Comments are closed.