Chemistry Of Soaps And Salts How Does Soap Work Kidpid

Chemistry Of Soaps And Salts How Does Soap Work Kidpid Salt is necessary for a living being and also it makes food taste better when salt is added. now, as soap is also formed by making an acid react with a base, it is also considered to be a salt. detergents have special molecules that have two parts, one is called the hydrophilic part and the other is called the hydrophobic part. Soap is a salt in chemistry, soap is a type of salt. this is because it is formed from the mixing of an acid and a base. how does soap work? soaps and detergents help to clean clothes, skin, dirty dishes, and other items by dissolving grease. detergents are made up of special molecules. part of these molecules is attracted to water.

Chemistry Of Soaps And Salts How Does Soap Work Kidpid In this collection of activities, students develop their understanding of key chemical ideas relating to soaps and detergents. suitable for 11–16 year olds, the resources draw on a variety of scientific, historical and everyday contexts, with activities ranging from making and testing soap to product analysis. stimulate and engage your. How soap works. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. in water, the sodium or potassium ions float free, leaving a negatively charged head. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. this combination creates clusters of soap, water, and grime called micelles. soap is a product that most of us use every day, yet most of. When you wash your hands, the soap forms something like a molecular bridge between the water and the dirty, germ laden oils on your hands, attaching to both the oils and the water and lifting the grime off and away. soaps can also link up with the fatty membranes on the outside of bacteria and certain viruses, lifting the infectious agents off.

Chemistry Of Soaps How Does Soap Work Chemystery 7 Surabhi Ma How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. this combination creates clusters of soap, water, and grime called micelles. soap is a product that most of us use every day, yet most of. When you wash your hands, the soap forms something like a molecular bridge between the water and the dirty, germ laden oils on your hands, attaching to both the oils and the water and lifting the grime off and away. soaps can also link up with the fatty membranes on the outside of bacteria and certain viruses, lifting the infectious agents off. Detergents. detergents are a family of compounds that are similar to soaps and work in a similar way. they are more useful in areas where hard water is present.this happens where there are high. Once the saponification reaction is complete, sodium chloride is added to precipitate the soap. the water layer is drawn off the top of the mixture and the glycerol is recovered using vacuum. the crude soap obtained from the saponification reaction contains sodium chloride, sodium hydroxide, and glycerol. these impurities are removed by boiling.
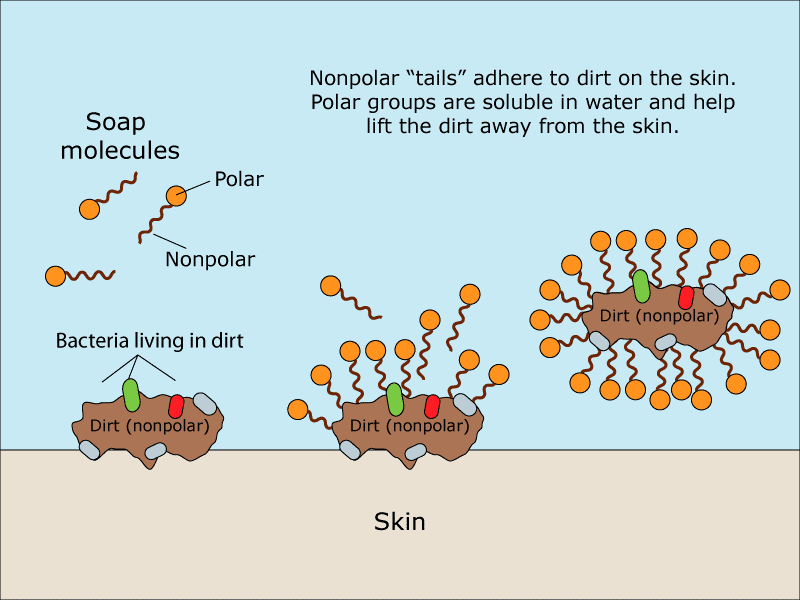
How Does Soap Actually Work Detergents. detergents are a family of compounds that are similar to soaps and work in a similar way. they are more useful in areas where hard water is present.this happens where there are high. Once the saponification reaction is complete, sodium chloride is added to precipitate the soap. the water layer is drawn off the top of the mixture and the glycerol is recovered using vacuum. the crude soap obtained from the saponification reaction contains sodium chloride, sodium hydroxide, and glycerol. these impurities are removed by boiling.

Comments are closed.