Chemistry Lab 14 Pdf Experiment 14 Acids Bases Salts And Buff

Experiment 14 Pdf Acids Bases Salts And Buffer Acids It also contains minerals and salts that were added to it. this may have affected our results when completing the titration with tap water. also while titrating, we may have added too much of the acid or base and that may have influenced our results. lab #14 post lab focus questions. focus questions 1. Experiment 14: acids, bases, salts and buffers “acid and bases are phun!!!” introduction. although we may not realize it we encounter acids, bases, salts, and buffer solutions. every single day. acids can be found in the items we consume, different areas of our body and in. the laboratory setting.
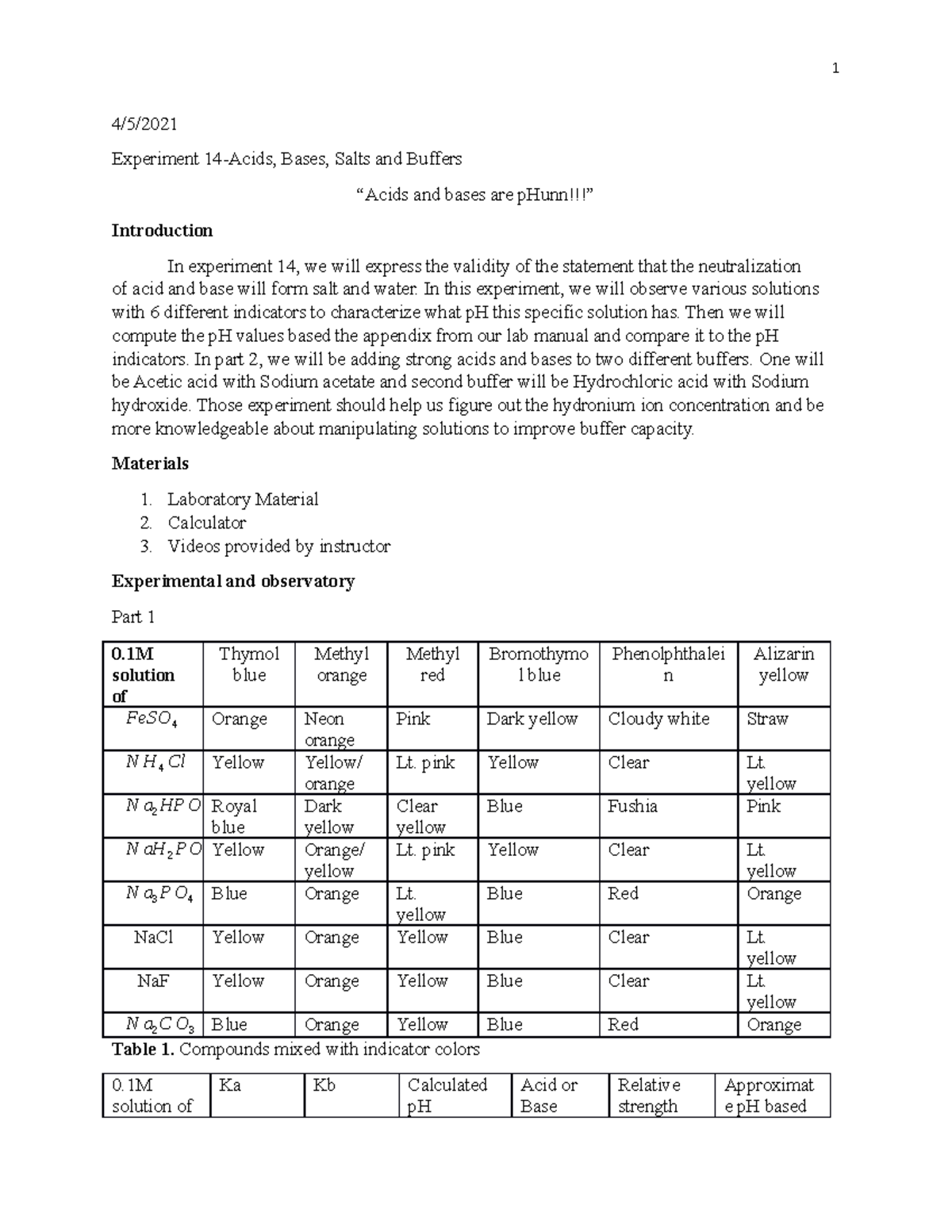
Experiment 14 Acids And Bases Are Phunn 4 5 Experiment 14 Aci A salt results from a neutralization reaction where acids and bases combine. as the h from the acid and oh ions from the base will form water, and the remaining acid and bases form a salt. a buffer is a solution of a weak acid and its conjugate weak base, which helps resist changes to its ph when small quantities of a base or acid are needed. Bicarbonate salts with acid. • write chemical equations for the reactions observed. advance preparation 6m hydrochloric acid dilute concentrated hcl 1:1 with distilled water. other materials cut a sufficient number of 2 cm long pieces of copper wire, iron wire (small nails may also be used), and chapter 19 • acids, bases, and salts experiment. Use 1 ml of salt solution. mix two together. make two solutions of weak acid and weak base. measure ph of each. dilute the the buffer solution and re measure ph. measure weak acid out 1 ml of or base. save mixtures for. Ph value = x [h ] = 10−xm (8.2) (8.2) ph value = x [h ] = 10 − x m. so for ph 7, the h h ion concentration is 10 7 m. the ph values of everyday chemicals typically range from ph 0 to ph 14. values between 0 and 7 indicate an acidic solution. values between 7 and 14 indicate a basic solution.

Lab 14 Chelsea Plamm Chem 106 10 Professor Raheem 4 April 2019 Lab Use 1 ml of salt solution. mix two together. make two solutions of weak acid and weak base. measure ph of each. dilute the the buffer solution and re measure ph. measure weak acid out 1 ml of or base. save mixtures for. Ph value = x [h ] = 10−xm (8.2) (8.2) ph value = x [h ] = 10 − x m. so for ph 7, the h h ion concentration is 10 7 m. the ph values of everyday chemicals typically range from ph 0 to ph 14. values between 0 and 7 indicate an acidic solution. values between 7 and 14 indicate a basic solution. Base is a donor of an electron pair. acid is an acceptor of an electron pair. for a species to function as a lewis acid, it needs to have an accessible empty orbital. for a species to function as a lewis base it needs to have an accessible electron pair. examples of lewis acids: bf3, alcl3, sbf5, na , h , s6 , etc. 1. ac. ids, bases, buffers and ph. purpose: introduction. acids and bases. arrhenius proposed the first theory of acids and bases. according to arrhenius, pure water dissociates to some extent to produce hydrogen ions (h ) and hydroxide ions (oh ). when this occurs, equal amounts of h and oh ions are produced:.
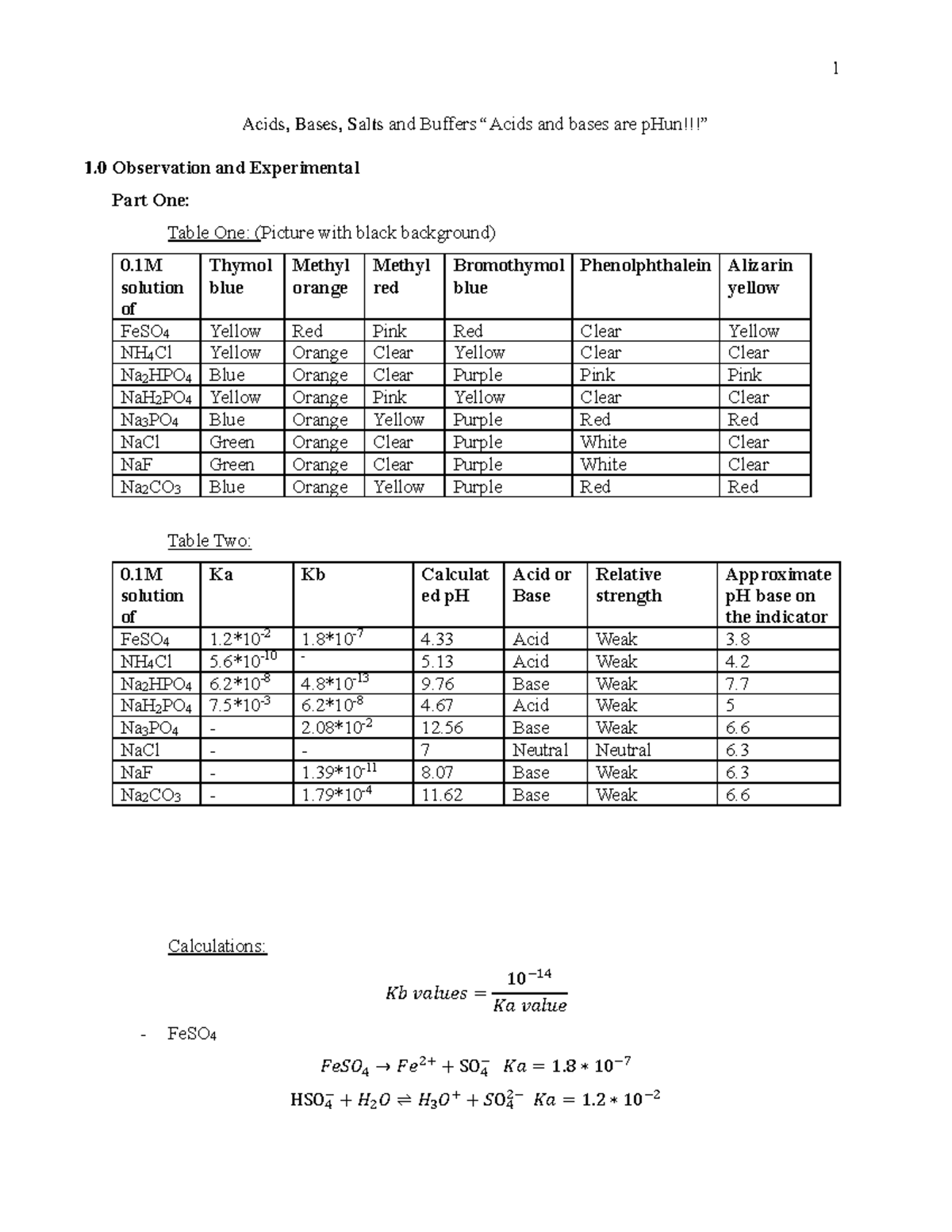
Lab Report 14 Acids Bases Salts And Buffers Acids And Basesођ Base is a donor of an electron pair. acid is an acceptor of an electron pair. for a species to function as a lewis acid, it needs to have an accessible empty orbital. for a species to function as a lewis base it needs to have an accessible electron pair. examples of lewis acids: bf3, alcl3, sbf5, na , h , s6 , etc. 1. ac. ids, bases, buffers and ph. purpose: introduction. acids and bases. arrhenius proposed the first theory of acids and bases. according to arrhenius, pure water dissociates to some extent to produce hydrogen ions (h ) and hydroxide ions (oh ). when this occurs, equal amounts of h and oh ions are produced:.

Comments are closed.