Chemistry 101 Calculating Pressure Volume Work

Chemistry 101 Calculating Pressure Volume Work Youtube Learning objective: learn to calculate pressure volume work from the changes in volume.topics: pressure volume work. Explanation for calculating work due to the expansion or compression of a gas.

Calculating Pressure Volume Work For A Chemical Reaction The piston moves as the molecules of the gas rapidly equilibrate to the applied pressure such that the internal and external pressures are the same. the result of this motion is work: wvolume = ∫(f a) (ads) = ∫ pdv (19.2.1) this particular form of work is called pressure volume (pv) work and will play an important role in the development of. If you're seeing this message, it means we're having trouble loading external resources on our website. if you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Learn how to calculate pressure volume work (pv work) for chemical reaction. this is a common exam problem in general chemistry thermodynamics.there are tons. The pressure volume work is: w = pΔv. the equation, w = pΔv, is the work done when a gas is expanded. the negative sign indicates that work is done on the surroundings by the system. we can calculate the work done by the system for our example above. the gas has expanded from 9 to 10 liters. Δv = 10.0 mol – 9.0 mol = 1.0 mol.

Chemistry 101 Change In Internal Energy Heat And Pressure Volume Learn how to calculate pressure volume work (pv work) for chemical reaction. this is a common exam problem in general chemistry thermodynamics.there are tons. The pressure volume work is: w = pΔv. the equation, w = pΔv, is the work done when a gas is expanded. the negative sign indicates that work is done on the surroundings by the system. we can calculate the work done by the system for our example above. the gas has expanded from 9 to 10 liters. Δv = 10.0 mol – 9.0 mol = 1.0 mol. Steps to calculate pressure volume work of a system. step 1: read the problem carefully to identify the initial and final values for pressure and volume. step 2: if the work is performed. Solution. first we need to determine the change in volume, Δ v. a change is always the final value minus the initial value: Δ v = v final − v initial = 6.19 l − 3.44 l = 2.75 l. now we can use the definition of work to determine the work done: w = − p ext · Δ v = − (1.26 atm) (2.75 l) = −3.47 l·atm.
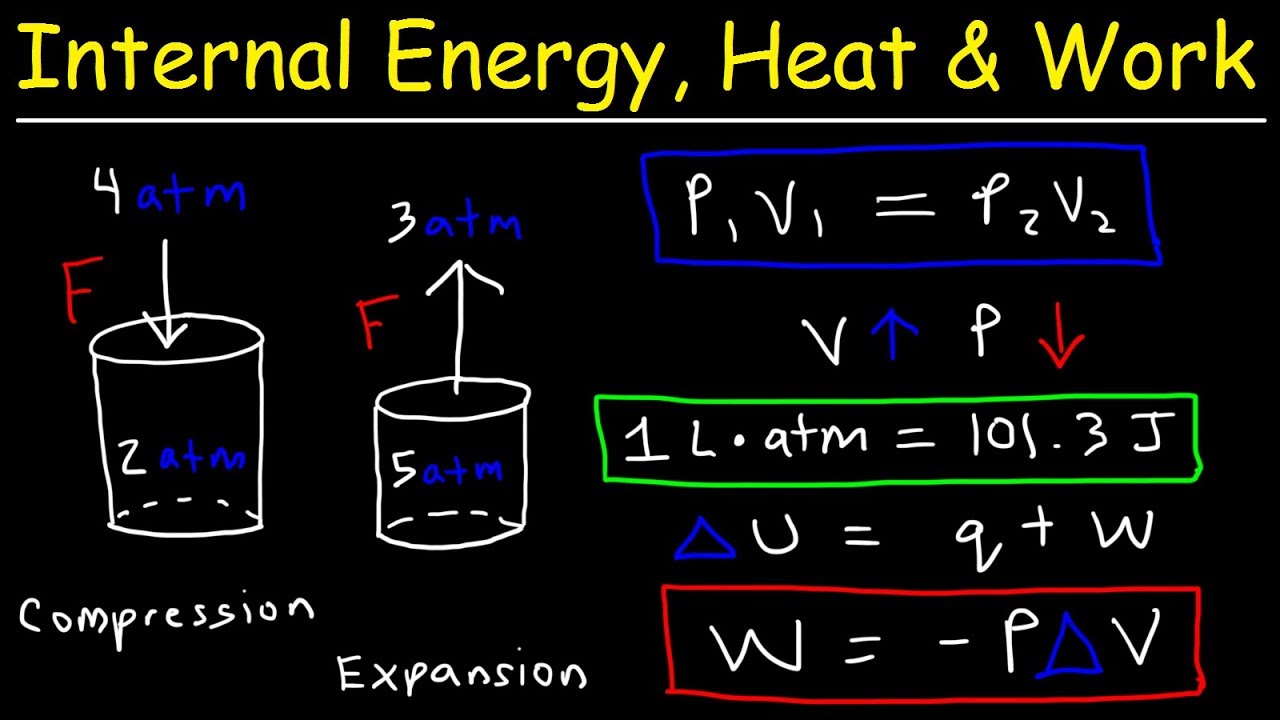
Examples Of Work Chemistry Steps to calculate pressure volume work of a system. step 1: read the problem carefully to identify the initial and final values for pressure and volume. step 2: if the work is performed. Solution. first we need to determine the change in volume, Δ v. a change is always the final value minus the initial value: Δ v = v final − v initial = 6.19 l − 3.44 l = 2.75 l. now we can use the definition of work to determine the work done: w = − p ext · Δ v = − (1.26 atm) (2.75 l) = −3.47 l·atm.

Pressure Volume Work Chemistry Youtube

Comments are closed.