Chemical Thermodynamics 3 2 Pressure Volume Work Old Version

Chemical Thermodynamics 3 2 Pressure Volume Work Youtube About press copyright contact us press copyright contact us. Short physical chemistry lecture on mechanical pressure volume work.when a gas expands against an external pressure, work is done on the surroundings, decrea.
.PNG)
Thermodynamics Presentation Chemistry Learn how to calculate pressure volume work (pv work) for chemical reaction. this is a common exam problem in general chemistry thermodynamics.there are tons. The whole system is considered as a machine, where you put heat inside, so that it must produce as much work as possible to be useful (δq> 0 δ q> 0). so the work done by the machine is positive if it works correctly, which happens if Δv Δ v is positive. for engineers, the work is defined as : δw = p Δv δ w = p Δ v. Figure 4. pressure–volume work. the system occupies the volume enclosed by the piston. if the cross sectional area of the cylinder is a a, and the system occupies a length x, x, the magnitude of the system’s volume is v = ax v = a x. if an applied pressure moves the piston a distance dx d x, the volume of the system changes by dvsystem = a. The piston moves as the molecules of the gas rapidly equilibrate to the applied pressure such that the internal and external pressures are the same. the result of this motion is work: wvolume = ∫(f a) (ads) = ∫ pdv (19.2.1) this particular form of work is called pressure volume (pv) work and will play an important role in the development of.
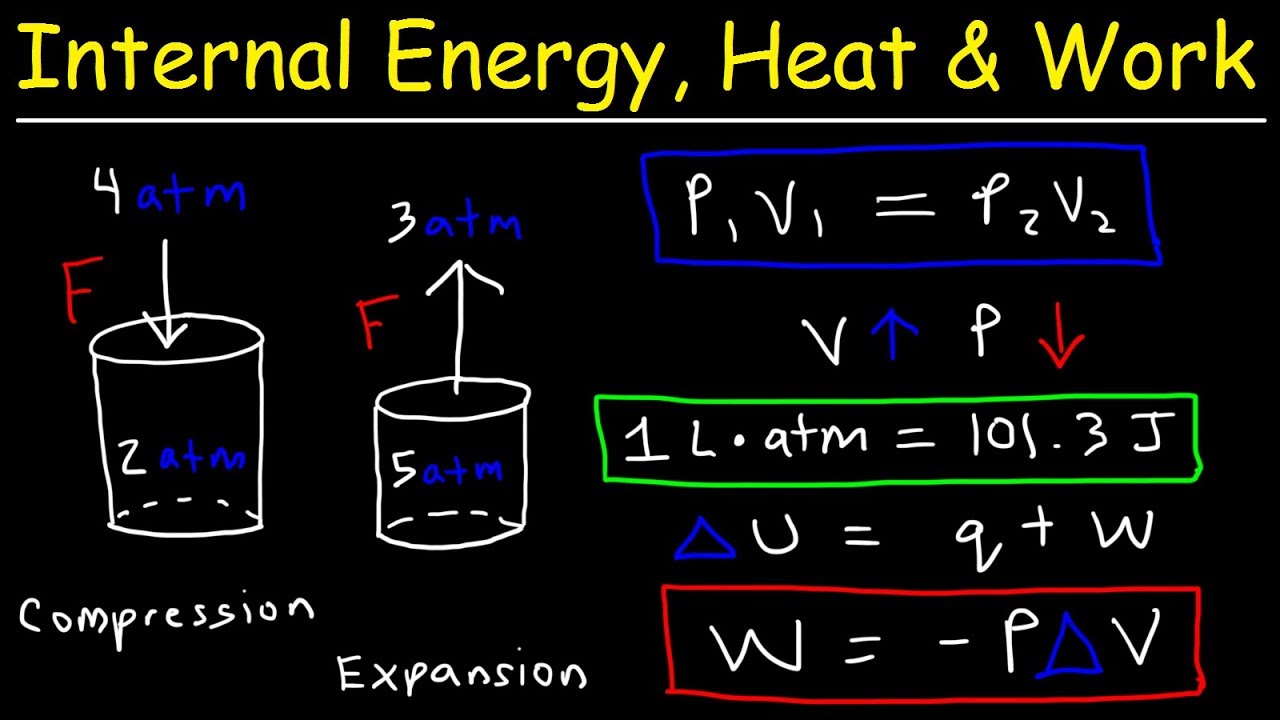
Pressure Volume And Temperature Equation Figure 4. pressure–volume work. the system occupies the volume enclosed by the piston. if the cross sectional area of the cylinder is a a, and the system occupies a length x, x, the magnitude of the system’s volume is v = ax v = a x. if an applied pressure moves the piston a distance dx d x, the volume of the system changes by dvsystem = a. The piston moves as the molecules of the gas rapidly equilibrate to the applied pressure such that the internal and external pressures are the same. the result of this motion is work: wvolume = ∫(f a) (ads) = ∫ pdv (19.2.1) this particular form of work is called pressure volume (pv) work and will play an important role in the development of. To achieve such an expansion, the external pressure is set equal to the pressure of the gas p at each stage of the expansion. then, − ∫vf vipdv. but when the system expands into vacuum, no work is done since system expands freely against zero external pressure, unlikely what you have mentioned. hence w = 0 since pext = 0,and not because p. The units of work obtained using this definition are correct for energy: pressure is force per unit area (newton m 2) and volume has units of cubic meters, so. w = (f a) Δv = newton m2 ×m3 w = (f a) Δ v = n e w t o n m 2 × m 3. figure 18.1.4 work performed with a change in volume the change in the volume (Δ v) of the cylinder housing a.

Comments are closed.