Chemical Reactions And Equations

How To Balance Chemical Equations 11 Steps With Pictures Learn how to write and balance chemical equations that describe chemical reactions. see examples of simple and complex reactions, and how to use coefficients and physical states to indicate the amounts of reactants and products. Learn about chemical reactions, the process of breaking and forming bonds between molecules to form new substances. explore different types of chemical reactions, such as combustion, decomposition, neutralization, redox and precipitation, with examples and equations.
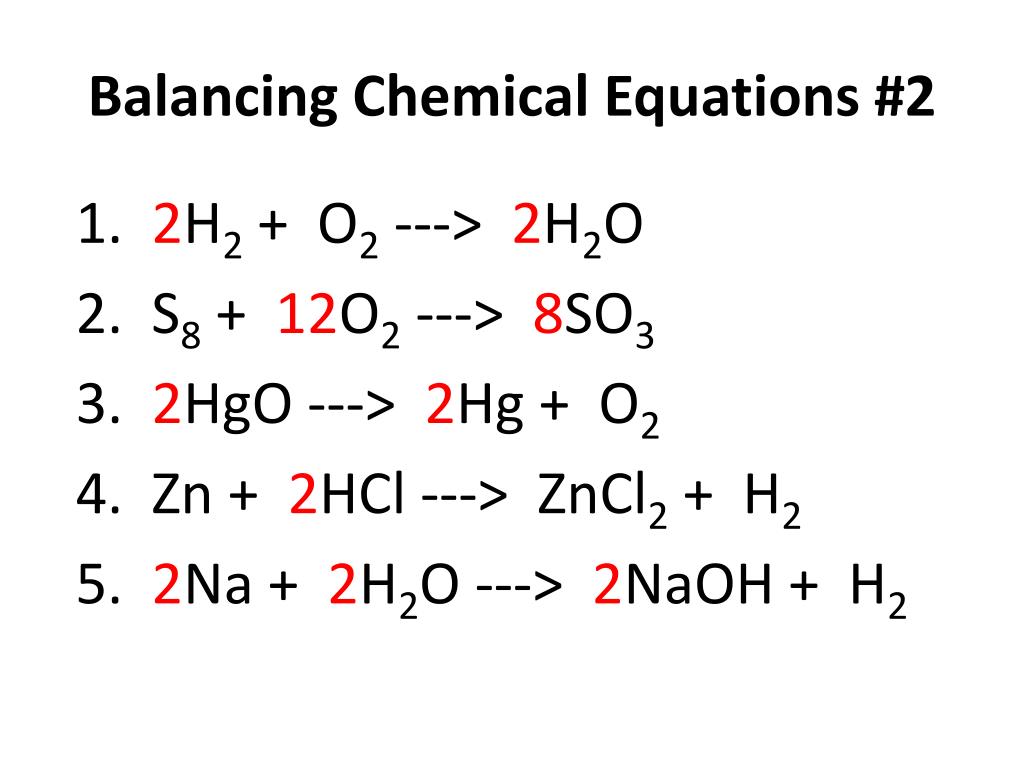
Balancing Chemical Equations Kahoot Chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. substances are either chemical elements or compounds. a chemical reaction rearranges the constituent atoms of the reactants to create different substances as products. chemical reactions are an integral. 7.3: chemical equations is shared under a not declared license and was authored, remixed, and or curated by libretexts. a chemical reaction is the process in which one or more substances are changed into one or more new substances. chemical reactions are represented by chemical equations. chemical equations have …. Therefore, we expect a reaction to occur, and the balanced chemical equation would be. na2so4(aq) srcl2(aq) → 2nacl (aq) srso4(s) you would expect to see a visual change corresponding to srso 4 precipitating out of solution ( figure 4.2 "double replacement reactions" ). figure 4.2 double replacement reactions. A balanced chemical equation includes coefficients before chemical formulas and indicates the stoichiometric ratio between reactants and products. a balanced chemical equation contains equal numbers and types of atoms on both sides of the reaction arrow. it is balanced for both mass and charge. example: 2h 2 o 2 → 2h 2 o.
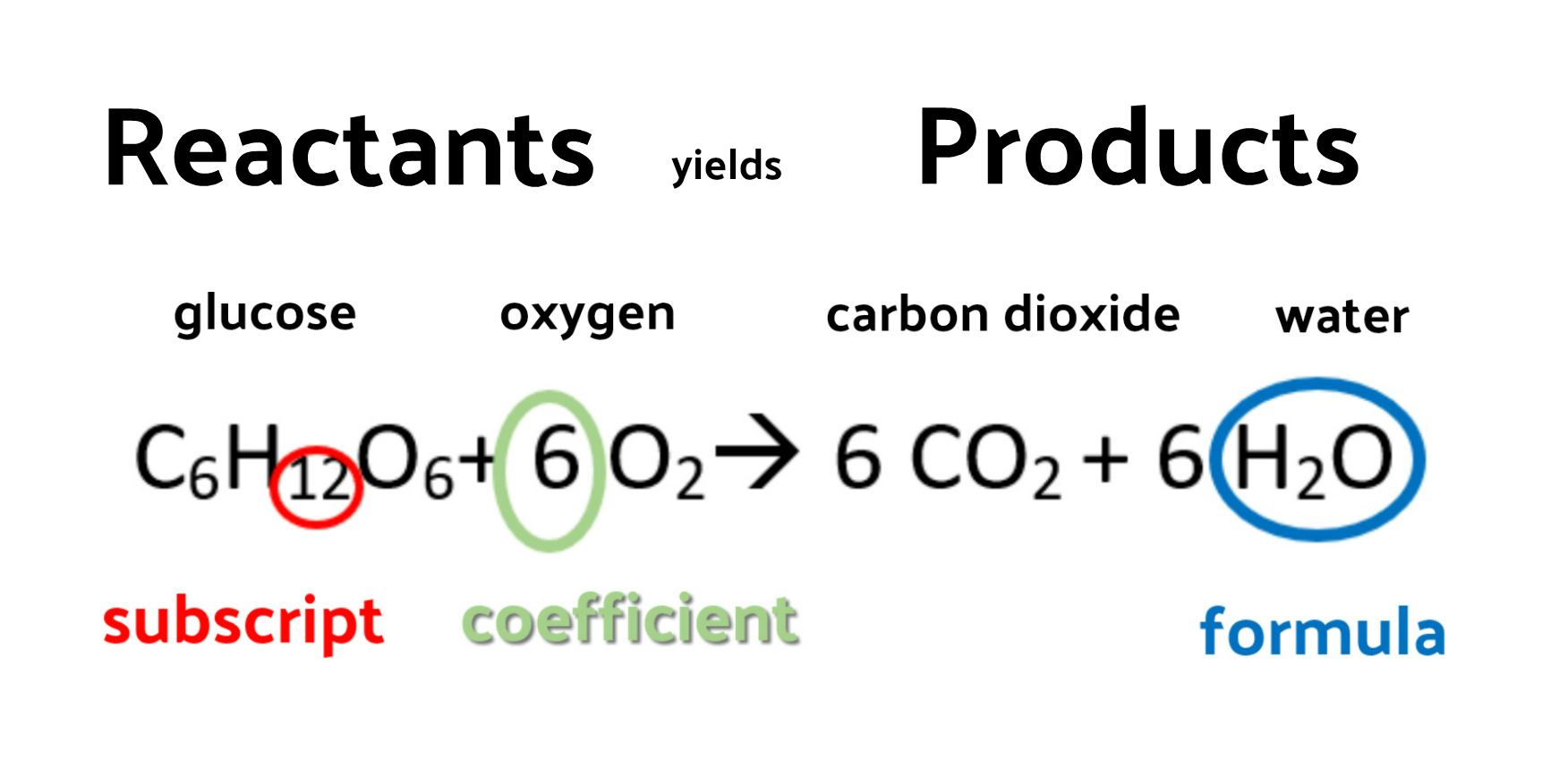
Chemical Reactions Balanced And Unbalanced Chemical Equations Online Therefore, we expect a reaction to occur, and the balanced chemical equation would be. na2so4(aq) srcl2(aq) → 2nacl (aq) srso4(s) you would expect to see a visual change corresponding to srso 4 precipitating out of solution ( figure 4.2 "double replacement reactions" ). figure 4.2 double replacement reactions. A balanced chemical equation includes coefficients before chemical formulas and indicates the stoichiometric ratio between reactants and products. a balanced chemical equation contains equal numbers and types of atoms on both sides of the reaction arrow. it is balanced for both mass and charge. example: 2h 2 o 2 → 2h 2 o. Step 4: the new count for each atom and polyatomic ion becomes: reactants products 1pbatom 1pbatom 2no − 3 ions 2no − 3 ions 2naatom 2naatom 2clatom 2clatoms. step 5: think about the result. the equation is now balanced since there are equal numbers of atoms of each element on both sides of the equation. Learn what chemical reactions are, how they are represented by equations, and how they involve bonds, energy, and products. watch a video and see questions and comments from other learners.
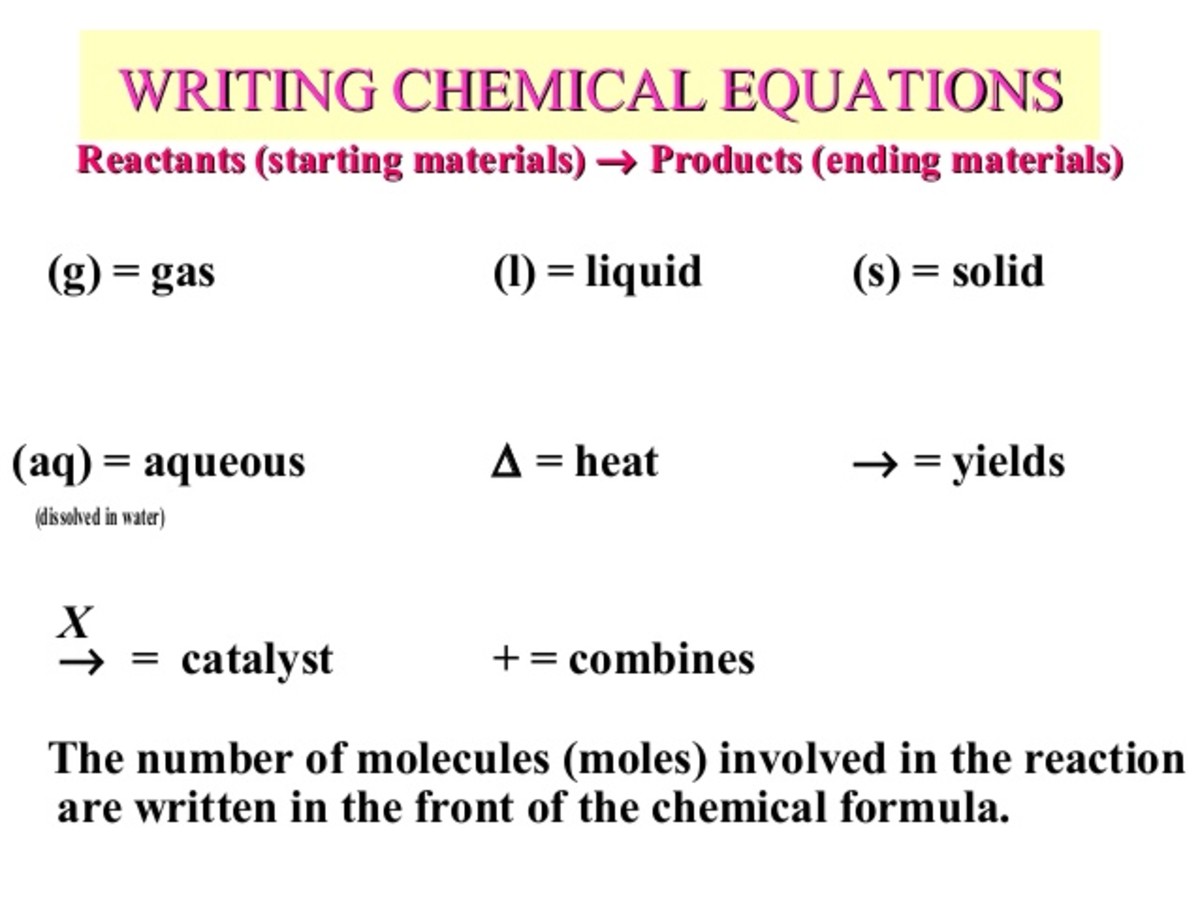
Chemical Reactions And Chemical Equations Owlcation Step 4: the new count for each atom and polyatomic ion becomes: reactants products 1pbatom 1pbatom 2no − 3 ions 2no − 3 ions 2naatom 2naatom 2clatom 2clatoms. step 5: think about the result. the equation is now balanced since there are equal numbers of atoms of each element on both sides of the equation. Learn what chemical reactions are, how they are represented by equations, and how they involve bonds, energy, and products. watch a video and see questions and comments from other learners.

Comments are closed.