Chemestry 11 Lessons Mole Conversions

Chemestry 11 Lessons Mole Conversions All assignments, notes & worksheets are to be submitted with the unit notebook. quiz corrections*: 1 2 mark for each correction. must be submitted with unit notebook. *completed on a separate page, questions written out in pen in full, explain why it was incorrect, explain how you corrected your mistake provide the correct answer in pencil. In chemistry, a mole is a very large number of things. typically, the this is a whiteboard animation tutorial of how to solve mole conversion calculations. in chemistry, a mole is a very large.

Mole Conversions Chem Worksheets 11 3 3 mol h 2 = 2 mol nh 3. prepare a concept map and use the proper conversion factor. cancel units and calculate. 4.20molh2 × 2molnh3 3molh2 = 2.80molnh3 4.20 mol h 2 × 2 mol n h 3 3 mol h 2 = 2.80 mol n h 3. the reaction of 4.20mol 4.20 mol of hydrogen with excess nitrogen produces 2.80mol 2.80 mol of ammonia. The conversion factor version! molar mass of c = 12.01g mol molar mass of mg = 24.3 g mol like saying 12in ft molar mass how many grams per mole? look on the periodic table! how much does a mole of something weigh??? 1 mole of c atoms = 1 mole of mg atoms = 1 mole of cu atoms = 12.0 g 24.3 g 63.5 g. 1 molal 26.98g al and 26.98gal 1mol al (5.4.1) (5.4.1) 1 m o l a l 26.98 g a l a n d 26.98 g a l 1 m o l a l. the first conversion factor can be used to convert from mass to moles, and the second converts from moles to mass. both can be used to solve problems that would be hard to do “by eye.”. It provides a specific measure of the number of atoms or molecules in a bulk sample of matter. a mole is defined as the amount of substance containing the same number of discrete entities (atoms, molecules, ions, etc.) as the number of atoms in a sample of pure 12c weighing exactly 12 g. one latin connotation for the word “mole” is “large.
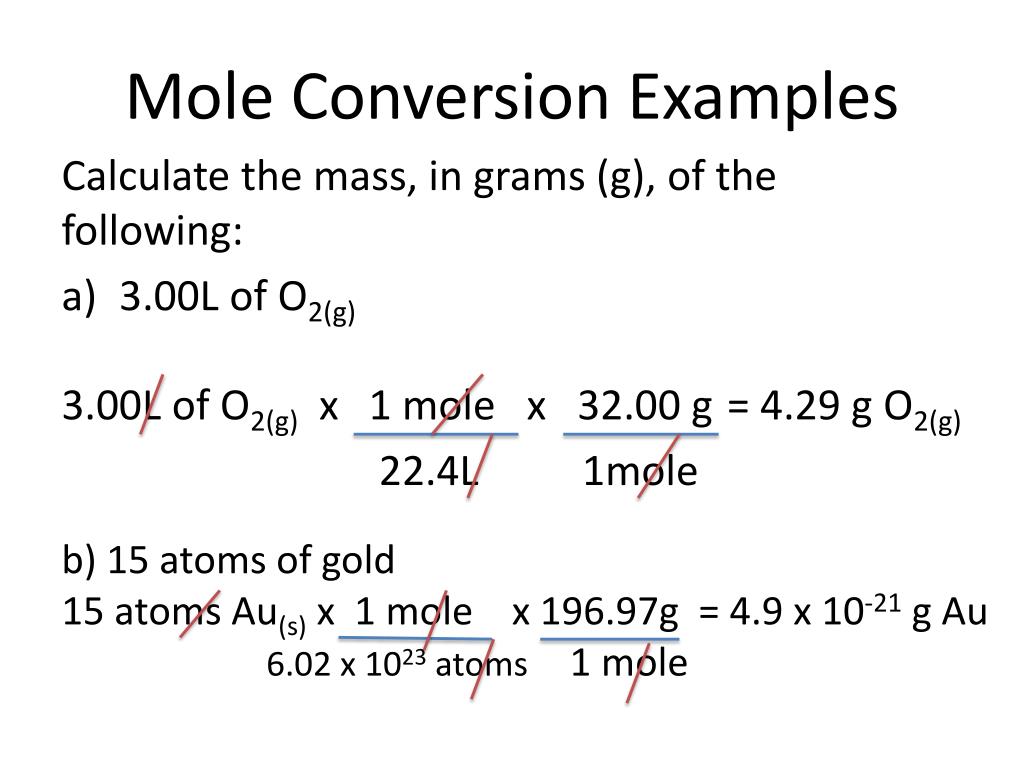
Chemistry Conversion Chart Moles 1 molal 26.98g al and 26.98gal 1mol al (5.4.1) (5.4.1) 1 m o l a l 26.98 g a l a n d 26.98 g a l 1 m o l a l. the first conversion factor can be used to convert from mass to moles, and the second converts from moles to mass. both can be used to solve problems that would be hard to do “by eye.”. It provides a specific measure of the number of atoms or molecules in a bulk sample of matter. a mole is defined as the amount of substance containing the same number of discrete entities (atoms, molecules, ions, etc.) as the number of atoms in a sample of pure 12c weighing exactly 12 g. one latin connotation for the word “mole” is “large. Step 1: list the known quantities and plan the problem. known. mass = 2.81 g. unknown. mol cu (oh) 2. one conversion factor will allow us to convert from mass to moles. step 2: calculate. first, it is necessary to calculate the molar mass of cu (oh) 2 from the molar masses of cu, o, and h. the molar mass is 97.57 g mol. Mole a mole of something is 6.02 × 10²³ of it. the mass of a mole of a substance is its relative mass expressed in grams. avogadro's constant avogadro's constant is the number of particles in one mole of a substance (6.02 × 10²³ mol⁻¹). relative formula mass the relative formula mass (rfm) of a substance is the sum of the.
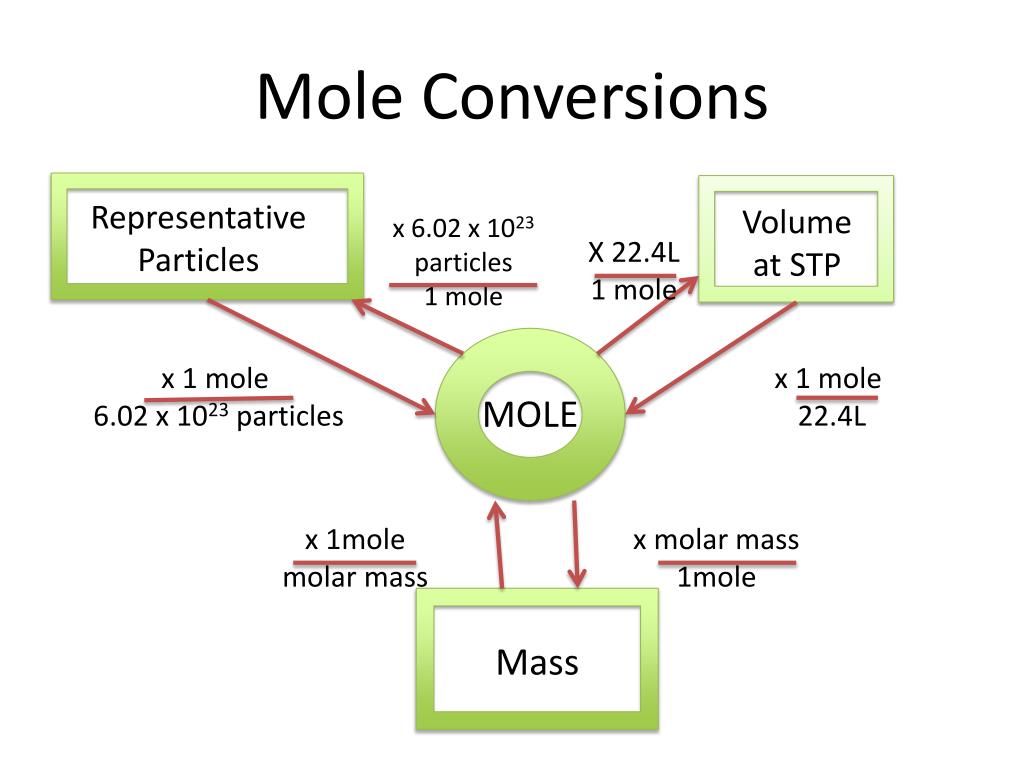
Mole Conversions Chem Worksheets 11 3 Step 1: list the known quantities and plan the problem. known. mass = 2.81 g. unknown. mol cu (oh) 2. one conversion factor will allow us to convert from mass to moles. step 2: calculate. first, it is necessary to calculate the molar mass of cu (oh) 2 from the molar masses of cu, o, and h. the molar mass is 97.57 g mol. Mole a mole of something is 6.02 × 10²³ of it. the mass of a mole of a substance is its relative mass expressed in grams. avogadro's constant avogadro's constant is the number of particles in one mole of a substance (6.02 × 10²³ mol⁻¹). relative formula mass the relative formula mass (rfm) of a substance is the sum of the.

Chemistry Conversion Chart Moles

Comments are closed.