Chem 111 Exam 1 F2210152022 Chem 111 Hfc Studocu
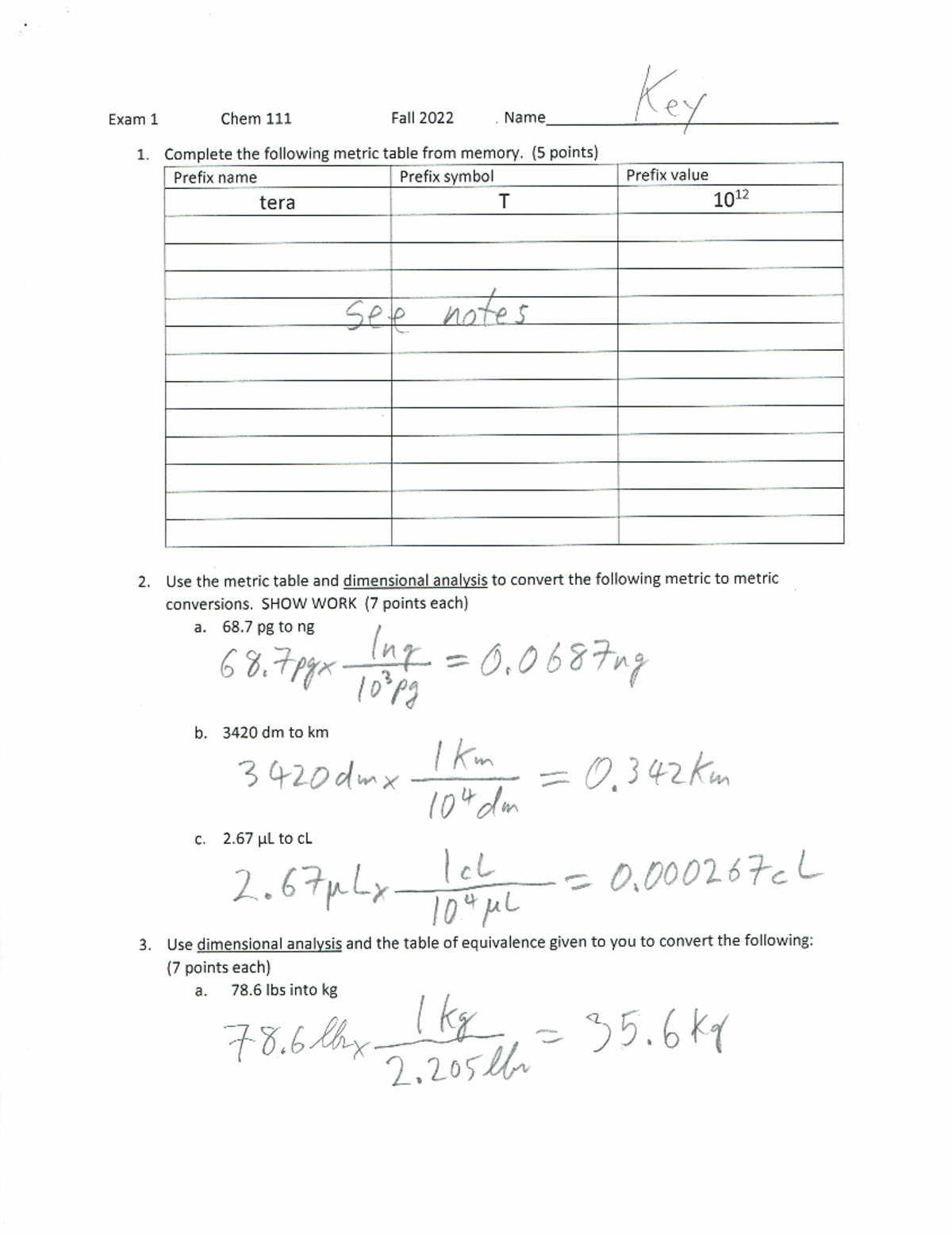
Chem 111 Exam 1 F2210152022 Chem 111 Hfc Studocu Welcome to studocu sign in to access the best study resources. 2022 2023. uploaded by: navine saleh. lab exam 1 chem 111 s22 student preview version. Exam 1 with answer state university of new york at binghamton, department of chemistry chemistry chemical principles, exam thursday february 2023 version fil in.

Chem 111 Exam 1 Summary Session Pdf Exam 1 Summary Session Ch Studying chem 111 chemical skills for pre professional programs at henry ford college? cam scanner 11 20 2022 15. 1 page. 2021 2022. none. 2021 chem 111 exam. 8. an isotope of an element has 14 protons, 14 electrons, and 14 neutrons. what is the mass? m= protons electrons. the production of pure aluminum foil bars of aluminum. physical change. study with quizlet and memorize flashcards containing terms like protons, nuetrons, electrons and more. Chapter 1 chemistry vocabulary (1.1) 1. define and use chemical vocabulary appropriately and accurately (for all chapters, all learning objectives, and all exams) accuracy: the closeness of measurement to the true or accepted value atomic mass unit (amu): a mass exactly equal to one twelfth the mass of one carbon 12 atom. Chem 111 exam 1. elements. click the card to flip 👆. the most basic unit of matter. substances composed of only one type of atom. cannot be broken down into smaller units by chemical means. ex: na, fe, o2, h2 (oxygen hydrogen don't exist in nature as h o) (elements are made up of atoms) click the card to flip 👆.
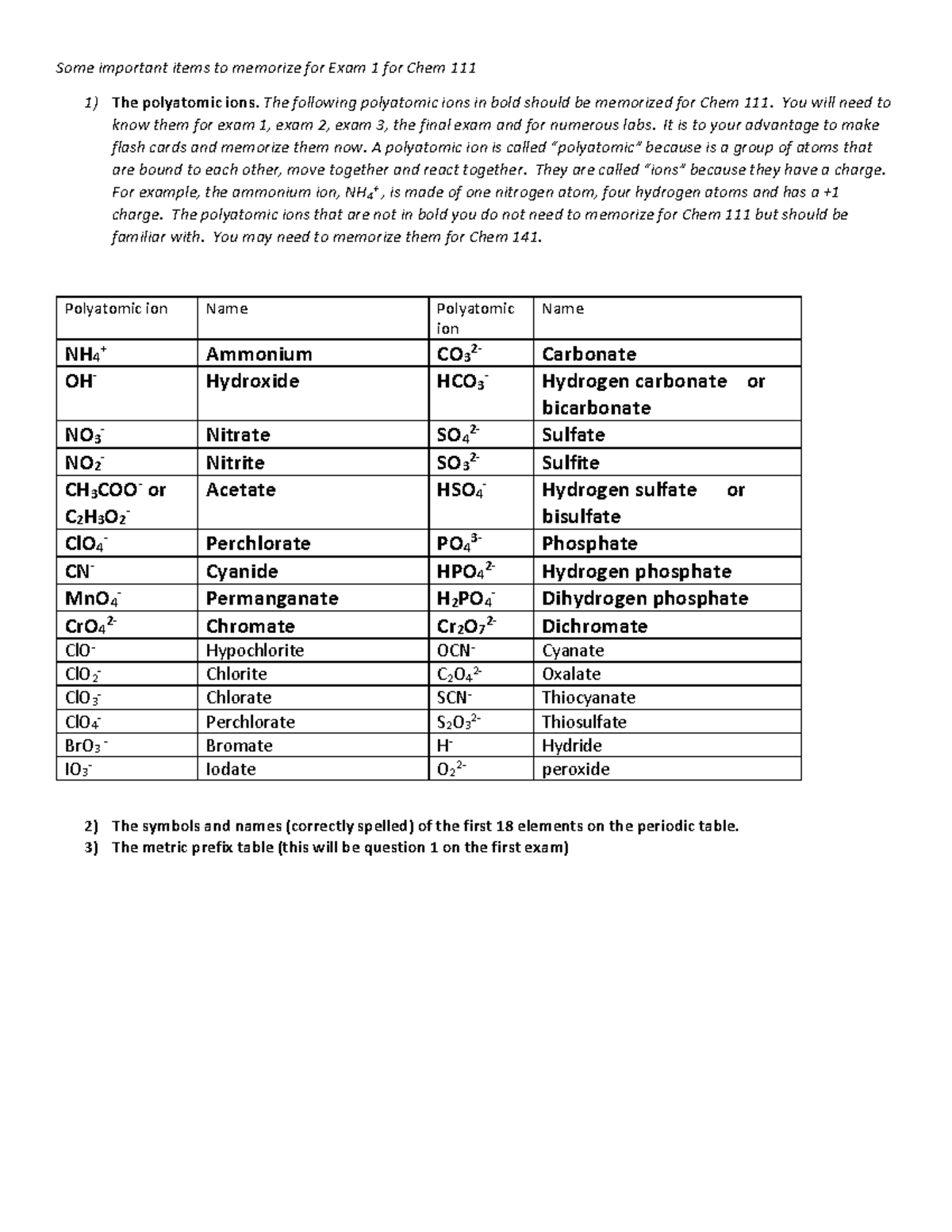
In Class Practice Problems Chem 111 W22 Some Important Items To Chapter 1 chemistry vocabulary (1.1) 1. define and use chemical vocabulary appropriately and accurately (for all chapters, all learning objectives, and all exams) accuracy: the closeness of measurement to the true or accepted value atomic mass unit (amu): a mass exactly equal to one twelfth the mass of one carbon 12 atom. Chem 111 exam 1. elements. click the card to flip 👆. the most basic unit of matter. substances composed of only one type of atom. cannot be broken down into smaller units by chemical means. ex: na, fe, o2, h2 (oxygen hydrogen don't exist in nature as h o) (elements are made up of atoms) click the card to flip 👆. A. water freezes at 273.15 k. b. hydrogen gas is an explosive. c. metals are reactive. a. water freezes at 273.15k. which of the following is an example of a chemical property? a. water boils at 100 degrees celsius. b. a copper penny weighs 3.00g. c.an iron nail rust. c. an iron nail rusts. Chem 111 study guide for exam 1: chapters 1 chapter 1: atoms. understand the difference among solids, liquids and gases. understand the composition of matter: elements, compounds, mixtures, etc. understand the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. understand the structure of atoms.

Lab Exam 2 Chem 111 S22 Sample Pdf Name Lab Exam 2 Chem 111 Summ A. water freezes at 273.15 k. b. hydrogen gas is an explosive. c. metals are reactive. a. water freezes at 273.15k. which of the following is an example of a chemical property? a. water boils at 100 degrees celsius. b. a copper penny weighs 3.00g. c.an iron nail rust. c. an iron nail rusts. Chem 111 study guide for exam 1: chapters 1 chapter 1: atoms. understand the difference among solids, liquids and gases. understand the composition of matter: elements, compounds, mixtures, etc. understand the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. understand the structure of atoms.

Chem 111 Exam I Study Guide Chem 111 Study Guide For Exam 1ођ

Comments are closed.