Chem 102 Experiment 8 Aldehyde Ketone Version Covid19 Pdf Aldehyde
Aldehydes Ketones Carboxylic Acids And Esters в Chemistry Figure 11.3.2 11.3. 2: esters are responsible for the odors associated with various plants and their fruits. both carboxylic acids and esters contain a carbonyl group with a second oxygen atom bonded to the carbon atom in the carbonyl group by a single bond. in a carboxylic acid, the second oxygen atom also bonds to a hydrogen atom. The compounds in figure 18.7.1 18.7. 1 are found chiefly in plants or microorganisms and those in figure 18.7.2 18.7. 2 have animal origins. aldehydes and ketones are known for their sweet and sometimes pungent odors. the odor from vanilla extract comes from the molecule vanillin.

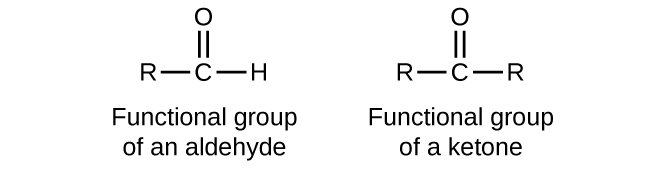
Tests For Aldehydes And Ketones Sodium Bisulphite Nahso3 2 4 Experiment 7 – aldehydes, ketones, and carboxylic acids. aldehydes and ketones are molecules that contain a carbonyl group, which is an oxygen atom with a double bond to a carbon atom. in an aldehyde, the carbonyl group is on the end of the molecule. in a ketone, the carbonyl group is somewhere in the middle of the molecule. Aldehydes and ketones, respectively (unit 7, class xii). 3. from hydrocarbons (i) by ozonolysis of alkenes: as we know, ozonolysis of alkenes followed by reaction with zinc dust and water gives aldehydes, 8.2.1 preparation of aldehydes and ketones 8.28.28.2 preparation of aldehydes and ketonesand ketones 5hsulqw. Aldehydes contain the carbonyl group bonded to at least one hydrogen atom. ketones contain the carbonyl group bonded to two carbon atoms. aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. the carbon atom of this group has two remaining bonds that may be occupied by hydrogen, alkyl or aryl. Identify aldehydes and ketones using brady’s reagent (2,4 dinitrophenylhydrazine) in this microscale experiment. in this practical, students add various liquid aldehydes and ketones to 2,4 dinitrophenylhydrazine solution in a well plate to form solid derivatives. they then do the same test with methanol and ethanol, showing that the reaction.
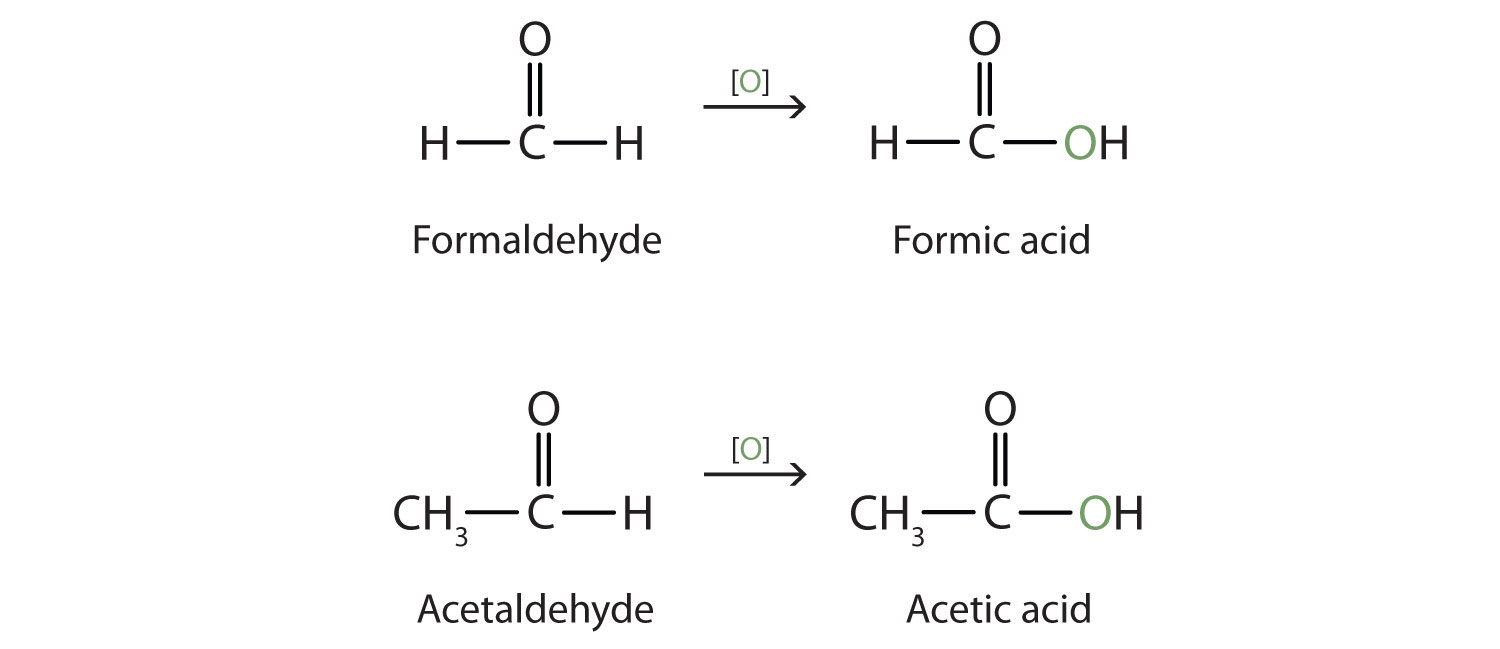
Chapter 3 Aldehydes Ketones Che 120 Introduction To Organic Aldehydes contain the carbonyl group bonded to at least one hydrogen atom. ketones contain the carbonyl group bonded to two carbon atoms. aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. the carbon atom of this group has two remaining bonds that may be occupied by hydrogen, alkyl or aryl. Identify aldehydes and ketones using brady’s reagent (2,4 dinitrophenylhydrazine) in this microscale experiment. in this practical, students add various liquid aldehydes and ketones to 2,4 dinitrophenylhydrazine solution in a well plate to form solid derivatives. they then do the same test with methanol and ethanol, showing that the reaction. In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. in a ketone, the carbonyl group is bonded to two carbon atoms: as text, an aldehyde group is represented as –cho; a ketone is represented as –c (o)– or –co–. in both aldehydes and ketones, the geometry around the carbon atom in the carbonyl group is trigonal. 14.4 reduction of aldehydes and ketones. in reduction reactions of aldehydes and ketones we add hydrogen across the double bond. that is, a hydrogen atom will be added to each atom of the double bond, converting the aldehyde. or ketone into an alcohol. we can add this hydrogen in one of two different ways.

As Chemistry Nomenclature Naming Aldehydes And Ketones Worksheets By In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. in a ketone, the carbonyl group is bonded to two carbon atoms: as text, an aldehyde group is represented as –cho; a ketone is represented as –c (o)– or –co–. in both aldehydes and ketones, the geometry around the carbon atom in the carbonyl group is trigonal. 14.4 reduction of aldehydes and ketones. in reduction reactions of aldehydes and ketones we add hydrogen across the double bond. that is, a hydrogen atom will be added to each atom of the double bond, converting the aldehyde. or ketone into an alcohol. we can add this hydrogen in one of two different ways.

Aldehydes Ketones Carboxylic Acids And Esters Chemistry Atoms First

Comments are closed.