Chem 008 Lab 03 Extraction Pdf Fig 3 1 Chem 008 Experiment 3

Chem 008 Lab 03 Extraction Pdf Fig 3 1 Chem 008 Experiment 3 Chem 008 lab 03 extraction page 1 of 8 author: dr. steve murov last revised 09 19 2021 by jml fig. 3 1 chem 008 experiment 3 extraction: a separation and purification technique (with an unexpected result) new techniques hippocrates (460 377 bc) used extract of willow bark and leaves for extraction, use of a separatory funnel. 1 fig. 3 1 chem 008 experiment 3 extraction: a separation and purification technique (with an unexpected result) new techniques hippocrates (460 377 bc) used extract of willow bark and leaves for extraction, use of a separatory funnel.
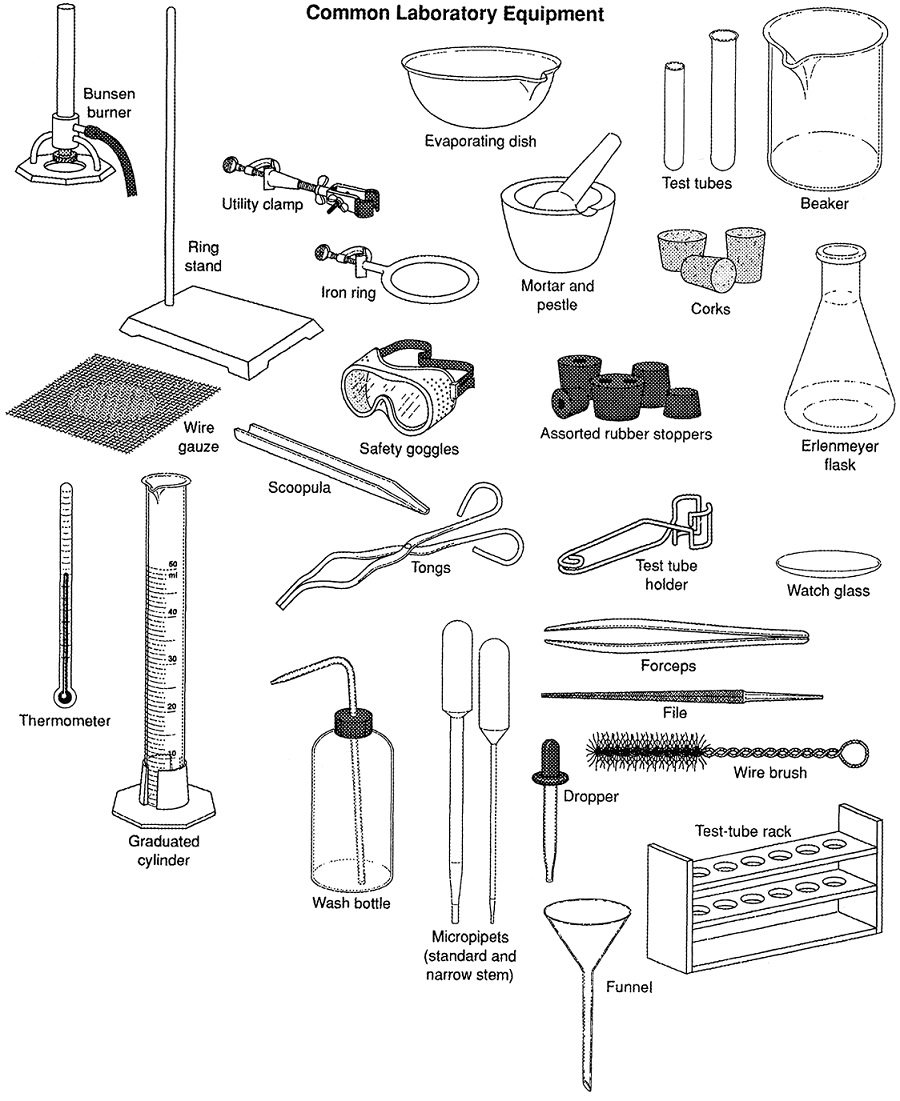
Common Laboratory Apparatus Chemistry Tutorial Chem 343 15 september 2020. experiment 3 lab report: extraction of acidic and neutral compounds. objective: separate desired products from an unknown mixture using the acidity and basicity of. the components of the mixture through a liquid liquid extraction. procedure observations 1. weigh out 500 mg of unknown mixture and record its mass. Ishanee kulkarni chem 2400 june 3 2020 experiment 3: extraction introduction: extraction is a widely used method by scientists for the separation of a substance from a mixture. it is frequently used to isolate a specific desired compound from a mixture or from its impurities. Preview text. experiment 3: extraction vanessa petit (300081874) section z04 ta: dmitry saraev procedure: refer to lab manual: “experiment 3: extraction”, chm 1321 organic chemistry laboratory manual, dr. rashmi venkateswaran , exp, p. 5 6. observations: part a: the methylene blue solution stayed in the aqueous part of the solution and the. We have chosen to extract the acidic compound into the aqueous layer using sodium hydroxide. outline the steps of the following procedure: place 3 g of the mixture in a 50 ml erlenmeyer flask and add 30 ml of diethyl ether. note: diethyl ether is highly flammable! care should be taken when handling this solvent.

Ochem Lab 3 Extraction Of A Three Component Mixture 1 Docx Lab 3 Preview text. experiment 3: extraction vanessa petit (300081874) section z04 ta: dmitry saraev procedure: refer to lab manual: “experiment 3: extraction”, chm 1321 organic chemistry laboratory manual, dr. rashmi venkateswaran , exp, p. 5 6. observations: part a: the methylene blue solution stayed in the aqueous part of the solution and the. We have chosen to extract the acidic compound into the aqueous layer using sodium hydroxide. outline the steps of the following procedure: place 3 g of the mixture in a 50 ml erlenmeyer flask and add 30 ml of diethyl ether. note: diethyl ether is highly flammable! care should be taken when handling this solvent. Duke university lab 3: extraction isolation of caffeine from tea 2 figure 2. examples of naturally occurring alkaloids. the challenge of today’s experiment is to separate the caffeine from the other components of tea (fig. 3): 1. cellulose: the major structural component of plants, including tea leaves. a high molecular weight, lin. Naphthalene is soluble in methanol and insoluble in water. it will dissolve in a hot mixture of 3:1 methanol:water, but is less soluble when this mixture is cold. to 4 recrystallize the naphthalene, heat about 50 ml of a mixture of 3:1 methanol:water almost to the boiling point on a steam bath.
Chemistry Pag 4 2 Learner V3 0 1 Duke university lab 3: extraction isolation of caffeine from tea 2 figure 2. examples of naturally occurring alkaloids. the challenge of today’s experiment is to separate the caffeine from the other components of tea (fig. 3): 1. cellulose: the major structural component of plants, including tea leaves. a high molecular weight, lin. Naphthalene is soluble in methanol and insoluble in water. it will dissolve in a hot mixture of 3:1 methanol:water, but is less soluble when this mixture is cold. to 4 recrystallize the naphthalene, heat about 50 ml of a mixture of 3:1 methanol:water almost to the boiling point on a steam bath.

Comments are closed.