Ch150 Chapter 7 Solutions Chemistry

Ch150 Chapter 7 Solutions Chemistry Chapter 7: solutions and solution stoichiometry 7.1 introduction 7.2 types of solutions 7.3 solubility 7.4 temperature and solubility 7.5 effects of pressure on the solubility of gases: henry’s law 7.6 solid hydrates 7.7 solution concentration 7.7.1 molarity 7.7.2 parts per solutions 7.8 dilutions 7.9 ion concentrations in solution 7.10 summary 7.11 references […]. The second part of the textbook focuses on ionic and covalent compounds and their nomenclature, an introduction to chemistry reactions, stoichiometry, and solutions chemistry. table of contents: chapter 1: measurements in chemistry chapter 2: atoms and the periodic table chapter 3: ions and ionic compounds chapter 4: covalent bonds and.

Ch150 Chapter 7 Solutions Chemistry Vrogue Co Chapter 7: solutions and solution stoichiometry 7.1 introduction 7.2 types of remedies 7.3 solution 7.4 temperature and solubility 7.5 influence of pressure on the solubility of fuels: henry’s law 7.6 solid hydrates 7.7 solution concentrates 7.7.1 molarity 7.7.2 accessories per products 7.8 oil 7.9 ion concentrations inside problem 7.10 summary 7.11 references […]. Chapter 5: chemical reactions this content can also be downloaded as an printable pdf, adobe reader is required for full functionality. this text is published under creative commons licensing, for referencing and adaptation, please click here. opening essay 5.1 the law of conservation of matter 5.2 writing and balancing chemical equations practice writing and balancing […]. Get solutions of icse class 10 concise chemistry selina chapter 7: metallurgy. clear your chemistry doubts instantly & get more marks in chemistry exam easily. master the concepts with our detailed explanations & solutions. Choose 7: solutions press solution stoichiometry 7.1 introduction 7.2 types away solutions 7.3 solubility 7.4 temperature both solubility 7.5 effects of pressure on the solubility about gases: henry’s law 7.6 solide hydrates 7.7 solution focused 7.7.1 molarity 7.7.2 parts per solutions 7.8 dilutions 7.9 iona concentrations included solution 7.10 summary 7.11 references […].

Ch150 Chapter 7 Solutions Chemistry Get solutions of icse class 10 concise chemistry selina chapter 7: metallurgy. clear your chemistry doubts instantly & get more marks in chemistry exam easily. master the concepts with our detailed explanations & solutions. Choose 7: solutions press solution stoichiometry 7.1 introduction 7.2 types away solutions 7.3 solubility 7.4 temperature both solubility 7.5 effects of pressure on the solubility about gases: henry’s law 7.6 solide hydrates 7.7 solution focused 7.7.1 molarity 7.7.2 parts per solutions 7.8 dilutions 7.9 iona concentrations included solution 7.10 summary 7.11 references […]. Question 7. it is required to reduce the volume of a gas by 20% by compressing it at a constant pressure. to do so, the gas has to be cooled. if the gas attains a final temperature of 157°c, find the initial temperature of the gas ? answer. initial conditions: v 1 = initial volume of the gas = v t 1 = initial temperature of the gas = ? final. Chapter 1 chapter 2 chapter 3 chapter 5 . material for test #2 . chapter 4 chapter 6, part a chapter 6, part b chapter 6, part c representing chemical reactions . material for test #3 . chapter 7 8 chapter 9. chapter 10. past exams (will be posted near test dates) test 1 test 1, key test 2 test 2, key test 3 test 3, key. suggested problems.
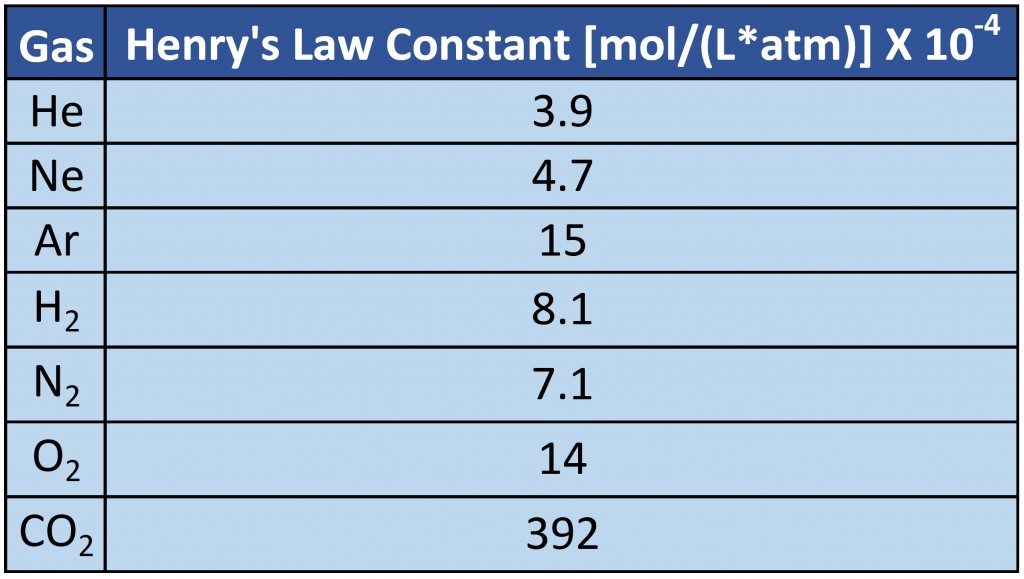
Ch150 Chapter 7 Solutions Chemistry Question 7. it is required to reduce the volume of a gas by 20% by compressing it at a constant pressure. to do so, the gas has to be cooled. if the gas attains a final temperature of 157°c, find the initial temperature of the gas ? answer. initial conditions: v 1 = initial volume of the gas = v t 1 = initial temperature of the gas = ? final. Chapter 1 chapter 2 chapter 3 chapter 5 . material for test #2 . chapter 4 chapter 6, part a chapter 6, part b chapter 6, part c representing chemical reactions . material for test #3 . chapter 7 8 chapter 9. chapter 10. past exams (will be posted near test dates) test 1 test 1, key test 2 test 2, key test 3 test 3, key. suggested problems.

Ch150 Chapter 7 Solutions Chemistry Vrogue Co

Comments are closed.