Cc A Painless Introduction To Vsepr Theory

Cc A Painless Introduction To Vsepr Theory A painless introduction to vsepr theory. today we will discuss vsepr (pronounced “vesper”), which stands for v alence s hell e lectron p air r epulsion. the basis of vsepr is that the electrons in bonds and lone pairs repel each other. to minimize the instability that results from these repulsions, a molecule will adopt the shape that. The valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of molecules. the theory is based on the idea of minimizing the electrostatic repulsion between electron pairs, as first proposed by sidgwick and powell in 1940, [9] then generalized by gillespie and nyholm in 1957, [10] and.

Cc A Painless Introduction To Vsepr Theory Because a lone pair is not shared by two nuclei, it occupies more space near the central atom than a bonding pair (figure 10.2.4 10.2. 4). thus bonding pairs and lone pairs repel each other electrostatically in the order bp–bp < lp–bp < lp–lp. in so 2, we have one bp–bp interaction and two lp–bp interactions. 4. The valence shell electron pair repulsion (vsepr) model predicts the shape of individual molecules based on the extent of electron pair electrostatic repulsion. according to vsepr, the valence electron pairs surrounding an atom mutually repel each other; they adopt an arrangement that minimizes this repulsion, thus determining the molecular. Contributed. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced "vesper") and is a model to predict the geometry of molecules. specifically, vsepr models look at the bonding and molecular geometry of organic molecules and polyatomic ions. it is useful for nearly all compounds that have a central atom that. When the two electron groups are 180° apart, the atoms attached to those electron groups are also 180° apart, so the overall molecular shape is linear. examples include beh 2 and co 2: figure 4.13.1 4.13. 1 beryllium hydride and carbon dioxide bonding. the two molecules, shown in the figure below in a "ball and stick" model.

Vsepr Theory Explanation Chart And Examples Contributed. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced "vesper") and is a model to predict the geometry of molecules. specifically, vsepr models look at the bonding and molecular geometry of organic molecules and polyatomic ions. it is useful for nearly all compounds that have a central atom that. When the two electron groups are 180° apart, the atoms attached to those electron groups are also 180° apart, so the overall molecular shape is linear. examples include beh 2 and co 2: figure 4.13.1 4.13. 1 beryllium hydride and carbon dioxide bonding. the two molecules, shown in the figure below in a "ball and stick" model. According to vsepr theory, a molecule is designated by the letters ax m e n. “a” represents the central atom, “x” represents the bonded atoms, “e” represents the lone pairs on the central atom, “m” is the number of electron groups or domains, and “n” is the number of lone pairs on the central atom. example: the water (h 2 o. 00:00 introduction01:03 geometric terminology02:15 tenets of vsepr theory04:10 electron pair geometries06:53 introducing molecular shape09:04 molecular shapes.
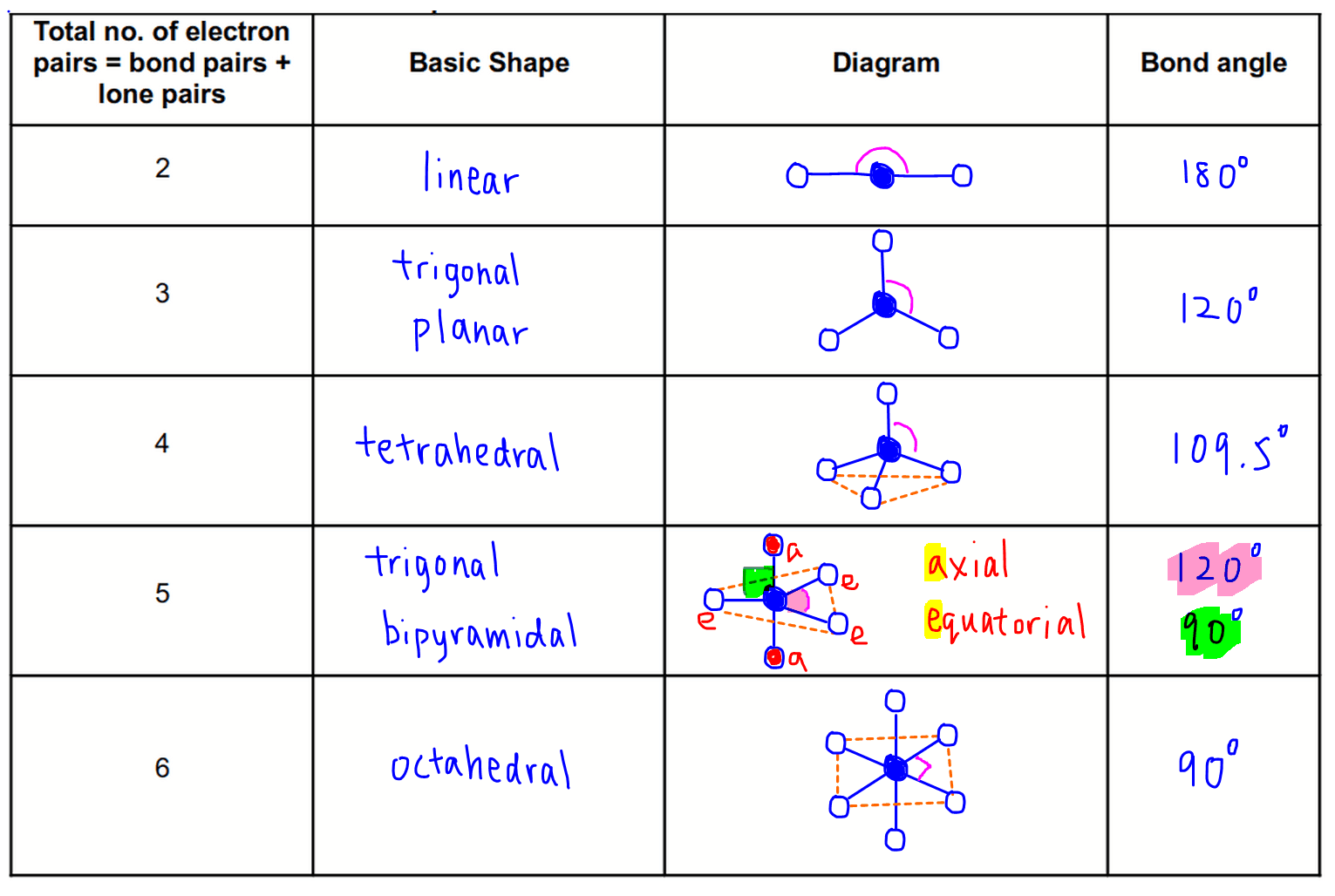
Vsepr Theory And Shapes Of Molecules According to vsepr theory, a molecule is designated by the letters ax m e n. “a” represents the central atom, “x” represents the bonded atoms, “e” represents the lone pairs on the central atom, “m” is the number of electron groups or domains, and “n” is the number of lone pairs on the central atom. example: the water (h 2 o. 00:00 introduction01:03 geometric terminology02:15 tenets of vsepr theory04:10 electron pair geometries06:53 introducing molecular shape09:04 molecular shapes.

Comments are closed.