Cation And Anion Diagram

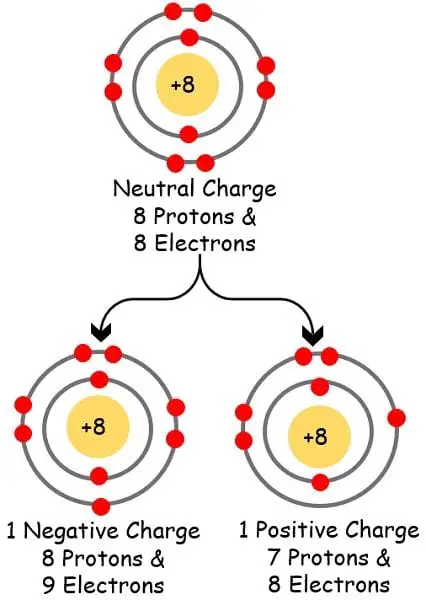
Cations And Anions Definitions Examples And Differences The letters in the word “anion” can stand for “a negative ion.” a pun to remember the difference is “cations are pawsitive.” writing chemical formulas. the chemical formula of a compound is always written with the cation first, followed by the anion. for example, na is the cation and cl is the anion in nacl (table salt). As you have learned, ions are atoms or molecules bearing an electrical charge. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell.
/cation-and-an-anion-differences-606111-v2_preview-5b44daf9c9e77c0037679d52.png)
The Difference Between A Cation And An Anion Anions and cations are both ions. they have an opposite electrical charge, therefore they get attracted to each other. cation repels other cation whereas anion repels another anion. the number of protons is more than the number of electrons in a cation whereas the number of electrons is more than the number of protons in an anion. Anion vs. cation. an ion is an atom or group of atoms in which the number of electrons is not equal to the number of protons, giving it a net positive or negative electrical charge. an anion is an ion that is negatively charged, and is attracted to the anode (positive electrode) in electrolysis. a cation has a net positive charge, and is. This video highlights the difference between cations and anions clearly explaining what they are and how they're made.chemistry basic introduction:. Cations are smaller than the parent atom because they've lost electrons. anions, on the other hand, gain new ones to become larger in size and have a positive charge. keywords. anion anode cathode cation electron lattice energy negatively ( ) charged ion nuclear charge polarity positively ( ) charged ion proton valence orbitals.
Difference Between Cation And Anion With Comparison Chart Bio This video highlights the difference between cations and anions clearly explaining what they are and how they're made.chemistry basic introduction:. Cations are smaller than the parent atom because they've lost electrons. anions, on the other hand, gain new ones to become larger in size and have a positive charge. keywords. anion anode cathode cation electron lattice energy negatively ( ) charged ion nuclear charge polarity positively ( ) charged ion proton valence orbitals. Cation vs anion periodic table. it can be possible to predict whether an atom will form a cation or an anion based on its position on the periodic table. halogens always form anions, alkali metals and alkaline earth metals always form cations. most other metals form cations (e.g. iron, silver, nickel), whilst most other nonmetals typically form. Polyatomic ions may be either positive or negative, for example: nh 4 (ammonium) = cation; so 4 2 (sulfate) = anion; the principles of ionic bonding with polyatomic ions are the same as those with monatomic ions. oppositely charged ions come together to form a crystalline lattice, releasing a lattice energy.

Comments are closed.