Birch Reduction Mechanism Procedure What Is Birch Reduction

Birch Reduction Definition Examples And Mechanism The birch reduction is a process for converting benzene (and its aromatic relatives) to 1,4 cyclohexadiene using sodium (or lithium) as a reducing agent in liquid ammonia as solvent (boiling point: –33°c) in the presence of an alcohol such as ethanol, methanol or t butanol. table of contents. the birch reduction. mechanism of the birch. The birch reduction is an organic reaction that is used to convert arenes to 1,4 cyclohexadienes.the reaction is named after the australian chemist arthur birch and involves the organic reduction of aromatic rings in an amine solvent (traditionally liquid ammonia) with an alkali metal (traditionally sodium) and a proton source (traditionally an alcohol).
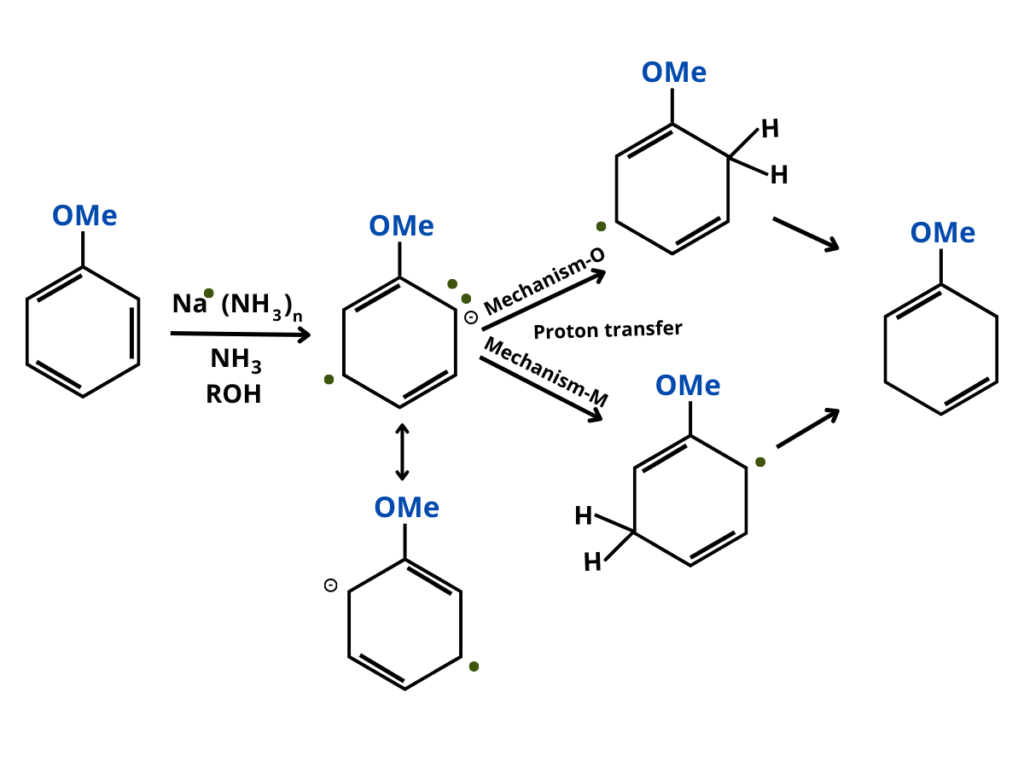
Birch Reduction Purechemistry Birch reduction is an organic redox reaction that is used to convert aromatic compounds into dienes. the reaction is carried out by sodium or potassium metal dissolved in liquid ammonia in the presence of alcohol. two hydrogen atoms are added at the opposite ends of the molecule at 1,4 positions. birds reaction can be applied in the total. The effect of electron withdrawing substituents on the birch reduction varies. for example, the reaction of benzoic acid leads to 2,5 cyclohexadienecarboxylic acid, which can be rationalized on the basis of the carboxylic acid stabilizing an adjacent anion: alkene double bonds are only reduced if they are conjugated with the arene, and. Here, an organic reduction of aromatic rings in liquid ammonia with sodium, lithium or potassium and alcohol occurs. an example of a birch reduction reaction is the reduction of naphthalene (illustrated below). birch reduction mechanism begins with the formation of the radical anion by the addition of solvated electrons to the aromatic ring. The birch reduction. another way of adding hydrogen to the benzene ring is by treatment with the electron rich solution of alkali metals, usually lithium or sodium, in liquid ammonia. see examples of this reaction, which is called the birch reduction. the birch reduction is the dissolving metal reduction of aromatic rings in the presence of an.
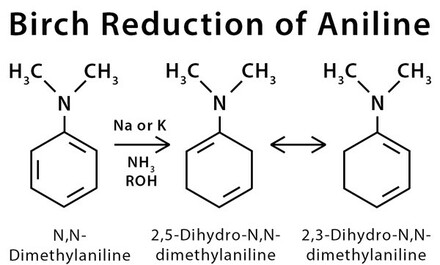
Birch Reduction Mechanism Procedure What Is Birch Reduction Here, an organic reduction of aromatic rings in liquid ammonia with sodium, lithium or potassium and alcohol occurs. an example of a birch reduction reaction is the reduction of naphthalene (illustrated below). birch reduction mechanism begins with the formation of the radical anion by the addition of solvated electrons to the aromatic ring. The birch reduction. another way of adding hydrogen to the benzene ring is by treatment with the electron rich solution of alkali metals, usually lithium or sodium, in liquid ammonia. see examples of this reaction, which is called the birch reduction. the birch reduction is the dissolving metal reduction of aromatic rings in the presence of an. Birch reduction of aromatic rings. description: the birch reduction is the reduction of an aromatic ring with sodium (or potassium) metal in ammonia (nh 3) in the presence of an alcohol. the result is a 1,4 cyclohexadiene. notes: don’t confuse this with nanh 2 nh 3, which is formation of a benzyne. The birch reduction is used to convert arenes to 1,4 cyclohexadienes. the reaction is named after the australian chemist arthur birch and involves the organic reduction of aromatic rings in an amine solvent (traditionally liquid ammonia) with an alkali metal (traditionally sodium) and a proton source (traditionally an alcohol).

Comments are closed.