Biomarkers In Drug Discovery And Development
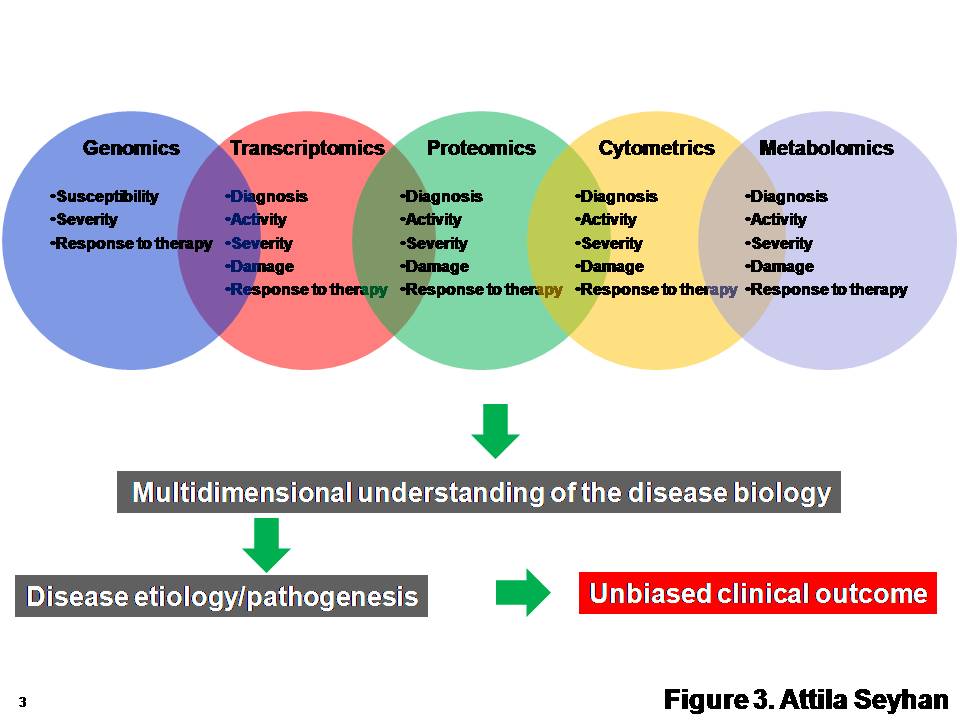
Biomarkers In Drug Discovery And Development This book continues the legacy of a well established reference within the pharmaceutical industry – providing perspective, covering recent developments in technologies that have enabled the expanded use of biomarkers, and discussing biomarker characterization and validation and applications throughout drug discovery and development.• explains where proper use of biomarkers can. A generic process of biomarker discovery and development begins with the analysis of a large number of biomarkers (analytes) and or tests in a small number of samples and culminates in the.

Biomarkers In Drug Discovery And Development By Using The Proteomic As both drug development and biomarker development are extremely cost and time intensive, it might be challenging for a sponsor to develop both simultaneously. 16 the current regulatory landscape for biomarkers progresses rapidly, with ongoing developments for both publicly reported biomarkers and biomarkers used in individual development. The use of genomic approaches as biomarkers could, however, have an earlier impact on the efficiency and probability of success in the preclinical drug discovery process by identifying profiles. Abstract. biomarkers are important tools in medicines development and clinical practice. besides their use in clinical trials, such as for enrichment of patients, monitoring safety or response to treatment, biomarkers are a cornerstone of precision medicine. the european medicines agency (ema) emphasised the importance of the discovery. Biomarkers are widely used at every stage of drug discovery and development. utilisation of biomarkers has a potential to make drug discovery, development and approval processes more efficient. an overview of the current global regulatory landscape is presented in this article with particular emphasis on the validation and qualification of.

Biomarkers In Drug Discovery And Development 9781119187509 R Abstract. biomarkers are important tools in medicines development and clinical practice. besides their use in clinical trials, such as for enrichment of patients, monitoring safety or response to treatment, biomarkers are a cornerstone of precision medicine. the european medicines agency (ema) emphasised the importance of the discovery. Biomarkers are widely used at every stage of drug discovery and development. utilisation of biomarkers has a potential to make drug discovery, development and approval processes more efficient. an overview of the current global regulatory landscape is presented in this article with particular emphasis on the validation and qualification of. However, there is a scarcity of robust and valid biomarkers to accelerate the drug development process from pre clinical through all stages of clinical studies. in this article, a brief overview of current definitions, biomarker categories, challenges in biological and analytical validation, along with several clinical examples will be presented. The act adopts into law the formal process, developed by the fda, of qualification of drug development tools, including biomarkers and clinical outcome assessments, to increase the efficiency of clinical trials and encourage an era of molecular medicine. the fda and european medicines agency (ema) have developed similar processes for the.
Biomarkers Used In Drug Discovery Clinical Development Download However, there is a scarcity of robust and valid biomarkers to accelerate the drug development process from pre clinical through all stages of clinical studies. in this article, a brief overview of current definitions, biomarker categories, challenges in biological and analytical validation, along with several clinical examples will be presented. The act adopts into law the formal process, developed by the fda, of qualification of drug development tools, including biomarkers and clinical outcome assessments, to increase the efficiency of clinical trials and encourage an era of molecular medicine. the fda and european medicines agency (ema) have developed similar processes for the.
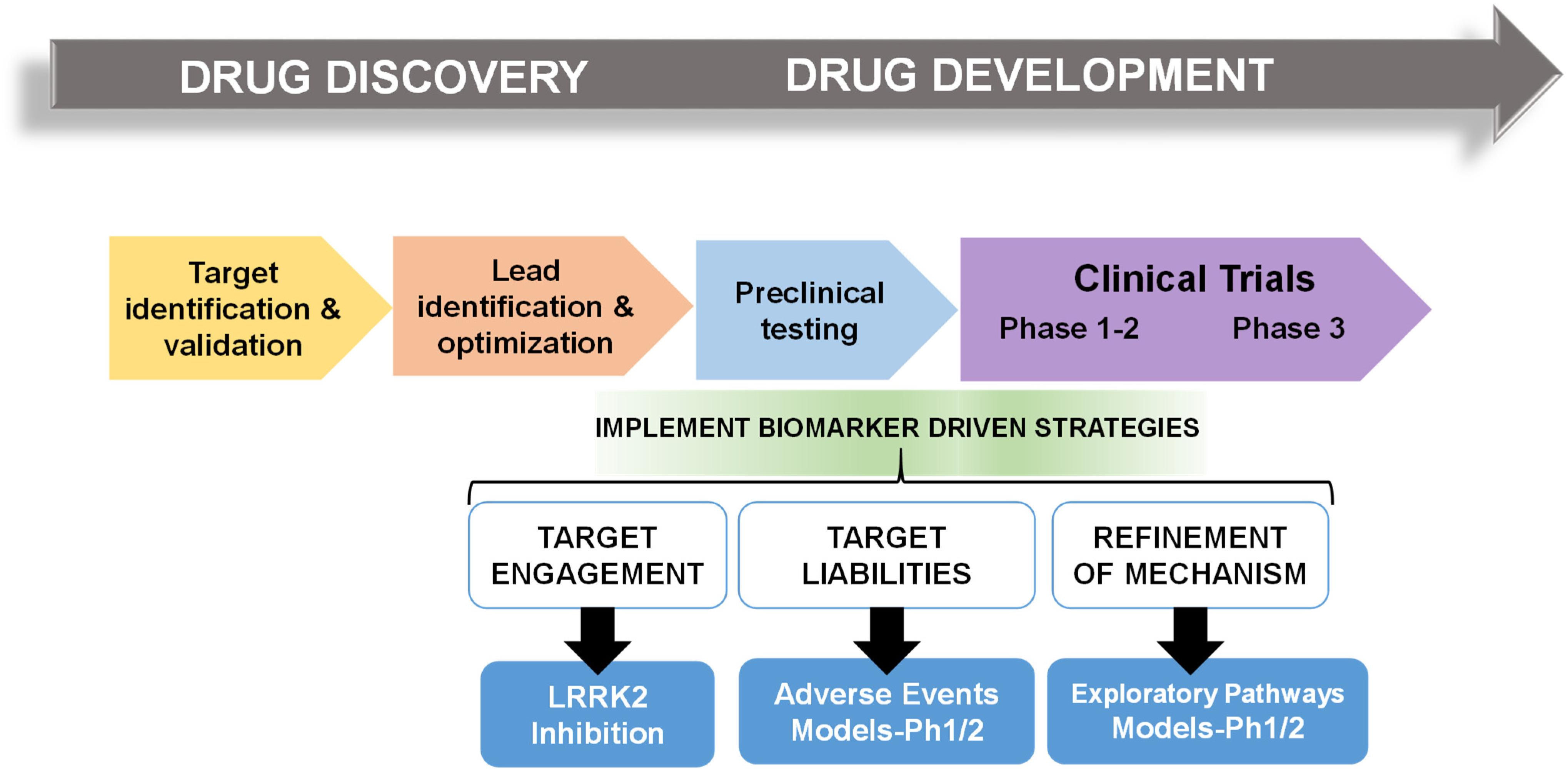
Frontiers Pharmacodynamic Biomarkers For Emerging Lrrk2 Therapeutics

Comments are closed.