Beryllium Chemical Element Of Mendeleev Periodic Table On Red

Beryllium Chemical Element Of Mendeleev Periodic Table On Red Eka tantalum is actually the synthetic superheavy element dubnium (105). mendeleev's 1869 table had implicitly predicted a heavier analog of titanium (22) and zirconium (40), but in 1871 he placed lanthanum (57) in that spot. the 1923 discovery of hafnium (72) validated mendeleev's original 1869 prediction. mendeleev [7] modern names. Today there are 111 elements recognized by iupac, and these are usually displayed in the form of a matrix called a periodic table. the term periodic came from the regular occurrence of certain chemical properties in the list of known elements when these are arranged in order of increasing relative mass. the common form, complete with the new.

Be Beryllium Data Periodic Table Mendeleev Stock Photo 1734037073 Mendeleev’s first periodic table in 1869 included the 63 known elements and spaces for three predicted, undiscovered elements. he revised and refined this table multiple times, as new data came to light. dmitri mendeleev did not invent the first periodic table. instead, he devised a table that organizes elements by atomic weight and periodic. Chemical element data in pubchem. pubchem is providing this periodic table page in order to help navigate abundant chemical element data available in pubchem. when exploring the table or list views on this page, please note the links to dedicated pages for each element. these individual element summary pages contain a lot of additional. Interactive periodic table showing names, electrons, and oxidation states. 4 be beryllium 9.0122; 5 b boron 10.81; for elements with no stable isotopes, the. Figure 2.5.1 2.5. 1: mendeleev’s periodic table, as published in the german journal annalen der chemie und pharmacie in 1872. the column headings “reihen” and “gruppe” are german for “row” and “group.”. formulas indicate the type of compounds formed by each group, with “r” standing for “any element” and superscripts.

The Element Beryllium In The Periodic Table Of Mendeleev Stock Interactive periodic table showing names, electrons, and oxidation states. 4 be beryllium 9.0122; 5 b boron 10.81; for elements with no stable isotopes, the. Figure 2.5.1 2.5. 1: mendeleev’s periodic table, as published in the german journal annalen der chemie und pharmacie in 1872. the column headings “reihen” and “gruppe” are german for “row” and “group.”. formulas indicate the type of compounds formed by each group, with “r” standing for “any element” and superscripts. Dmitri ivanovich mendeleev was a russian chemist and physicist, born on february 8, 1834 and died on february 2, 1907. he is best known for his contribution to the creation of the periodic table of elements, a fundamental tool for the study of chemistry. in 1869, mendeleev published his "periodic table of chemical elements" in which he. In order of increasing atomic mass, mendeleev thought about the elements beryllium, boron, carbon, nitrogen, oxygen, and fluorine. these elements were all different in their physical and chemical properties, thus seeming to belong to different families. mendeleev put their cards in a vertical row, with lithium at the. li be.
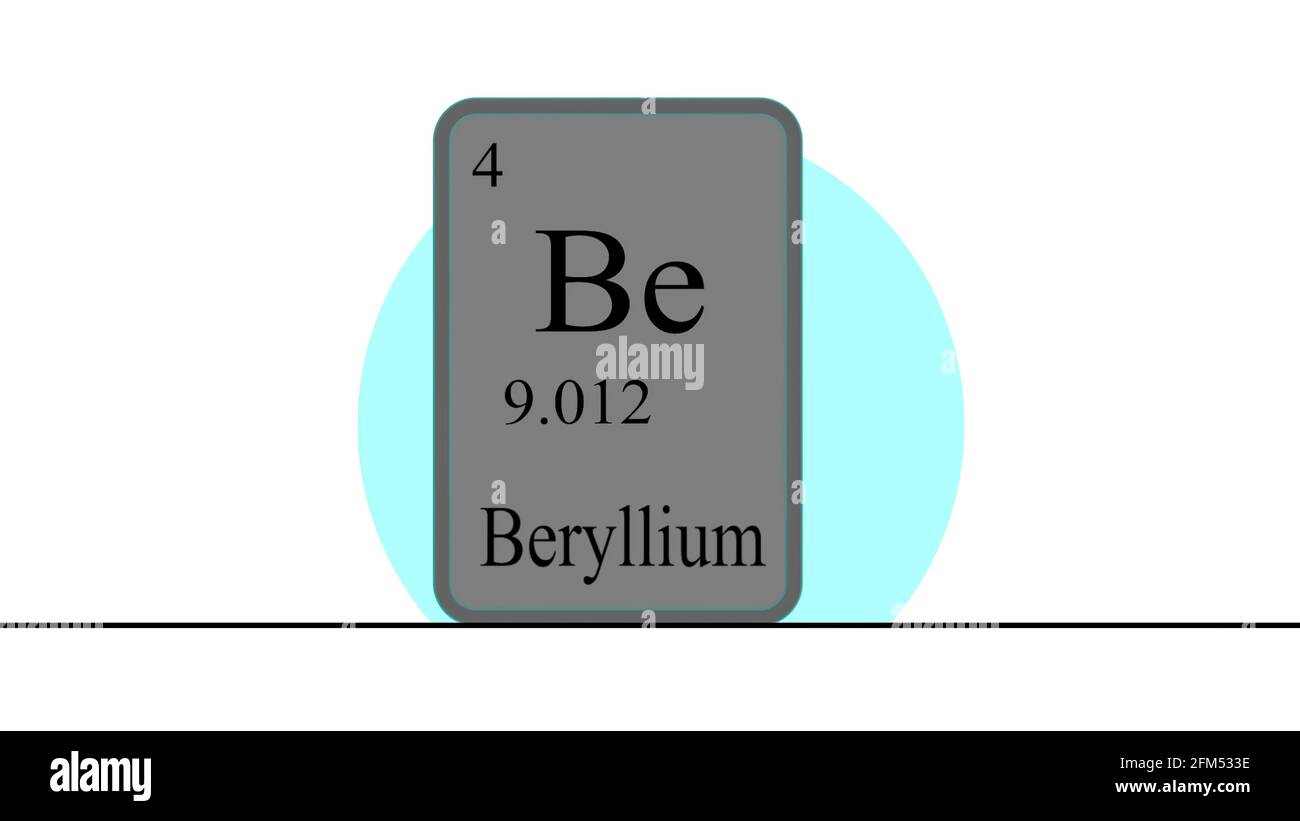
3d Illustration Beryllium Element Of The Periodic Table Of The Dmitri ivanovich mendeleev was a russian chemist and physicist, born on february 8, 1834 and died on february 2, 1907. he is best known for his contribution to the creation of the periodic table of elements, a fundamental tool for the study of chemistry. in 1869, mendeleev published his "periodic table of chemical elements" in which he. In order of increasing atomic mass, mendeleev thought about the elements beryllium, boron, carbon, nitrogen, oxygen, and fluorine. these elements were all different in their physical and chemical properties, thus seeming to belong to different families. mendeleev put their cards in a vertical row, with lithium at the. li be.

Comments are closed.