Avogadro S Number The Mole Grams Atoms Molar Mass Calculations

юааavogadroюабтащюааsюаб юааnumberюаб Converting Between юааatomsюаб And юааmolesюаб Youtube This general chemistry video tutorial focuses on avogadro's number and how it's used to convert moles to atoms. this video also shows you how to calculate t. To calculate this result, multiply 6 moles by avogadro's number: 6 × 6.02214076 × 10²³ = 3.613 × 10²⁴. molar mass calculator. this avogadro's number calculator will help you find the number of molecules in a mole.
.PNG)
Avogadro S Number And The Mole Avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. however, it also defines the mole, so we can also express na as 6.02 × 1023 mol–1; in this form, it is properly known as avogadro's constant. this construction emphasizes the role of avogadro's number as a conversion factor between number of moles and number of. Avogadro's number, the mole, grams, atoms, molar mass calculations introduction. Convert from mass to moles by dividing the mass given by the compound’s molar mass. convert from moles to molecules by multiplying the number of moles by avogadro’s number. solution: a the molecular mass of ethylene glycol can be calculated from its molecular formula using the method illustrated in example 3.4.1:. Solution: 1) each mole of molecules contains n number of molecules, where n equals avogadro's number. how many molecules are in each answer: (a) 1 x n = n (b) 2 x n = 2n (c) 4 x n = 4n (d) n x 5 = 5n. 2) each n times the number of hydrogen atoms in a formula equals the total number of hydrogen atoms in the sample:.
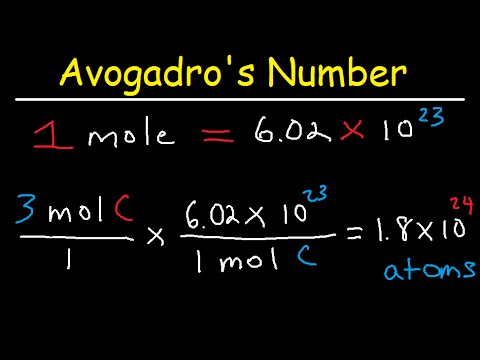
Avogadro S Number The Mole Grams Atoms Molar Mass Calculations Convert from mass to moles by dividing the mass given by the compound’s molar mass. convert from moles to molecules by multiplying the number of moles by avogadro’s number. solution: a the molecular mass of ethylene glycol can be calculated from its molecular formula using the method illustrated in example 3.4.1:. Solution: 1) each mole of molecules contains n number of molecules, where n equals avogadro's number. how many molecules are in each answer: (a) 1 x n = n (b) 2 x n = 2n (c) 4 x n = 4n (d) n x 5 = 5n. 2) each n times the number of hydrogen atoms in a formula equals the total number of hydrogen atoms in the sample:. The mole is defined as the amount of substance that contains the number of carbon atoms in exactly 12 g of carbon 12, avogadro’s number (6.022 × 10 23) of atoms of carbon 12. the molar mass of a substance is defined as the mass of 1 mol of that substance, expressed in grams per mole, and is equal to the mass of 6.022 × 10 23 atoms. Understand molar mass and its expression in grams per mole, enabling conversions between substance mass and molar quantities. practice calculations involving the number of atoms in a sample and determine the molar mass of compounds like co2.
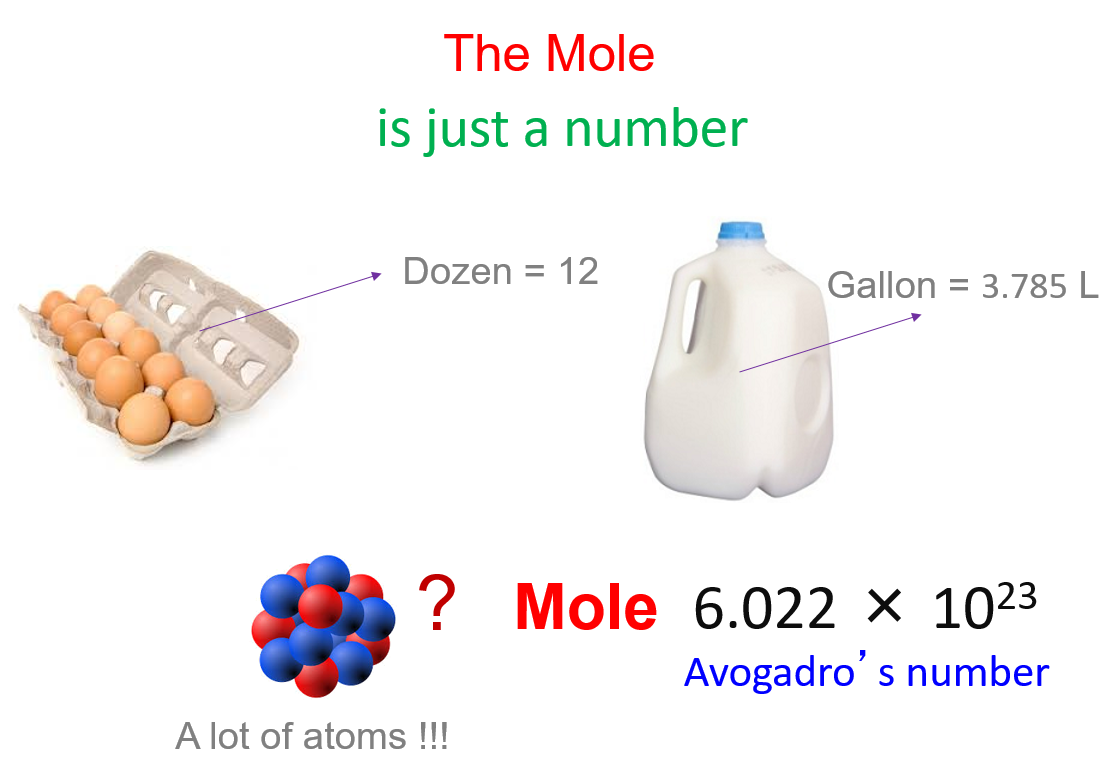
The Mole And Molar Mass Chemistry Steps The mole is defined as the amount of substance that contains the number of carbon atoms in exactly 12 g of carbon 12, avogadro’s number (6.022 × 10 23) of atoms of carbon 12. the molar mass of a substance is defined as the mass of 1 mol of that substance, expressed in grams per mole, and is equal to the mass of 6.022 × 10 23 atoms. Understand molar mass and its expression in grams per mole, enabling conversions between substance mass and molar quantities. practice calculations involving the number of atoms in a sample and determine the molar mass of compounds like co2.

Moles And Avogadro S Number Formula

Comments are closed.