Amines And Amino Acids Revise Im
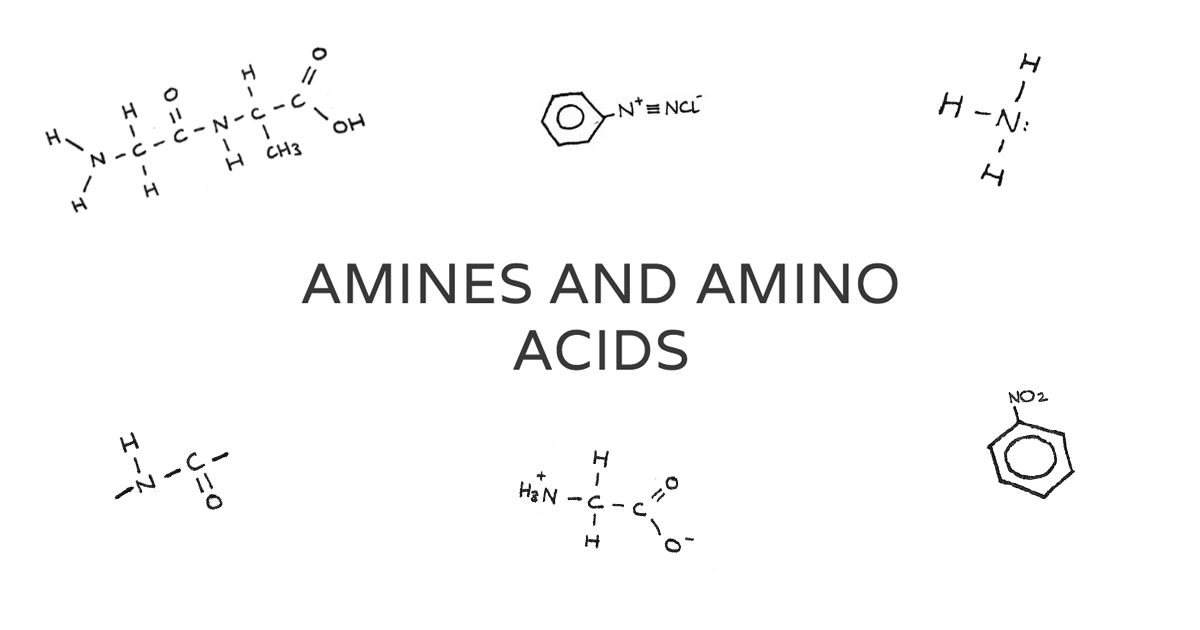
Amines And Amino Acids Revise Im Amino acids are amphoteric, meaning they can react as an acid or a base. at a ph more acidic than the isoelectric point, the amino acid behaves as a base and accepts a proton from the acid. this results in a positively charged ion. h 3 nch2coo− → h 3 nch2cooh. at a ph less acidic than the isoelectric point, the amino acid behaves as an acid. Amino acid. definition. a compound containing a nitrogen atom bonded to one or more carbon atoms. an organic compound that contains both an amino group ( nh2) and a carboxyl group ( cooh). chemical formula. can vary depending on the specific amine compound. generally written as nh2 chr cooh, where r represents a side chain.
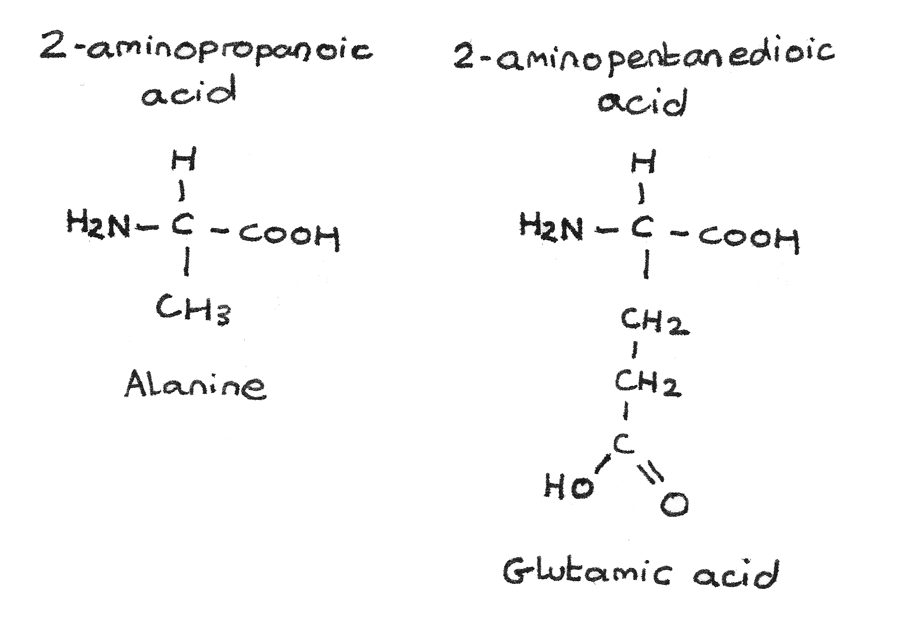
Amines And Amino Acids Revise Im All amino acids, except glycine, are chiral because there are four different groups around the c they rotate plane polarised light. h2n c co 2h ch3 h nh2 c ho c ch3 h some amino acids have an extra carboxylic acid or an amine group on the r group. these are classed as acidic or basic (respectively) amino acids nh2 c co2h ch2 h co2h aspartic. Revision notes on 5.5.1 amines, amides & amino acids introduction for the edexcel international a level chemistry syllabus, written by the chemistry experts at save my exams. 3.11 amines ch3nh2 h2o ch3nh3 oh nh3 (aq) h2o (l) nh4 (aq) oh (aq) primary aliphatic amines act as bronsted lowry bases because the lone pair of electrons on the nitrogen is readily available for forming a dative covalent bond with a h and so accepting a proton. primary aliphatic amines are stronger bases than ammonia as the. Since each of the original amino acids has an unreacted group (one has an unreacted amine and the other an unreacted carboxylic acid), more peptide bonds can form to other amino acids, extending the structure. (figure \(\pageindex{4}\)) a chain of connected amino acids is called a polypeptide. proteins contain at least one long polypeptide chain.
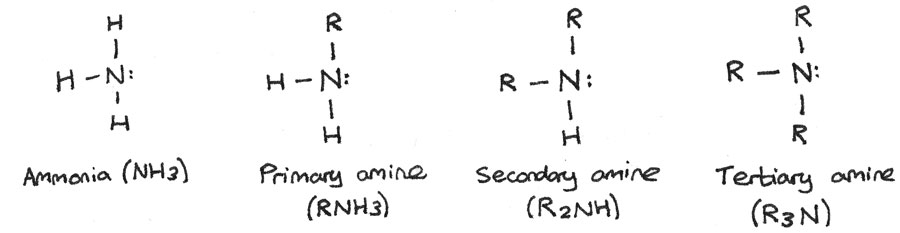
Amines And Amino Acids Revise Im 3.11 amines ch3nh2 h2o ch3nh3 oh nh3 (aq) h2o (l) nh4 (aq) oh (aq) primary aliphatic amines act as bronsted lowry bases because the lone pair of electrons on the nitrogen is readily available for forming a dative covalent bond with a h and so accepting a proton. primary aliphatic amines are stronger bases than ammonia as the. Since each of the original amino acids has an unreacted group (one has an unreacted amine and the other an unreacted carboxylic acid), more peptide bonds can form to other amino acids, extending the structure. (figure \(\pageindex{4}\)) a chain of connected amino acids is called a polypeptide. proteins contain at least one long polypeptide chain. E z isomerism is one form of stereoisomerism where atoms can have different orientations around a double bond. optical isomerism is another type of stereoisomerism, named due to how the isomers affect plane polarised light. it arises in organic molecules that contain a carbon atom bonded to four different atoms or groups. Some amino acids have an extra co2h carboxylic acid or an amine ch2 group on the r group. these are classed as acidic or basic nh2 c co2h. (respectively) amino acids h aspartic acid. co2h. h c (ch2)4 nh2 lycine (basic) h2n 2,6 diaminohexanoic acid. if the side r group of an amino acid contains an acidic of basic group then ph value of the.

Comments are closed.