Adiabatic Process Animation

Examples Of Adiabatic Processes This is an animation of a system gas that is cooling using the adiabatic process. Physics animations physics english adia txt.htmcompression and expansion of an adiabatically isolated gas is accompanied by its heating and coolin.
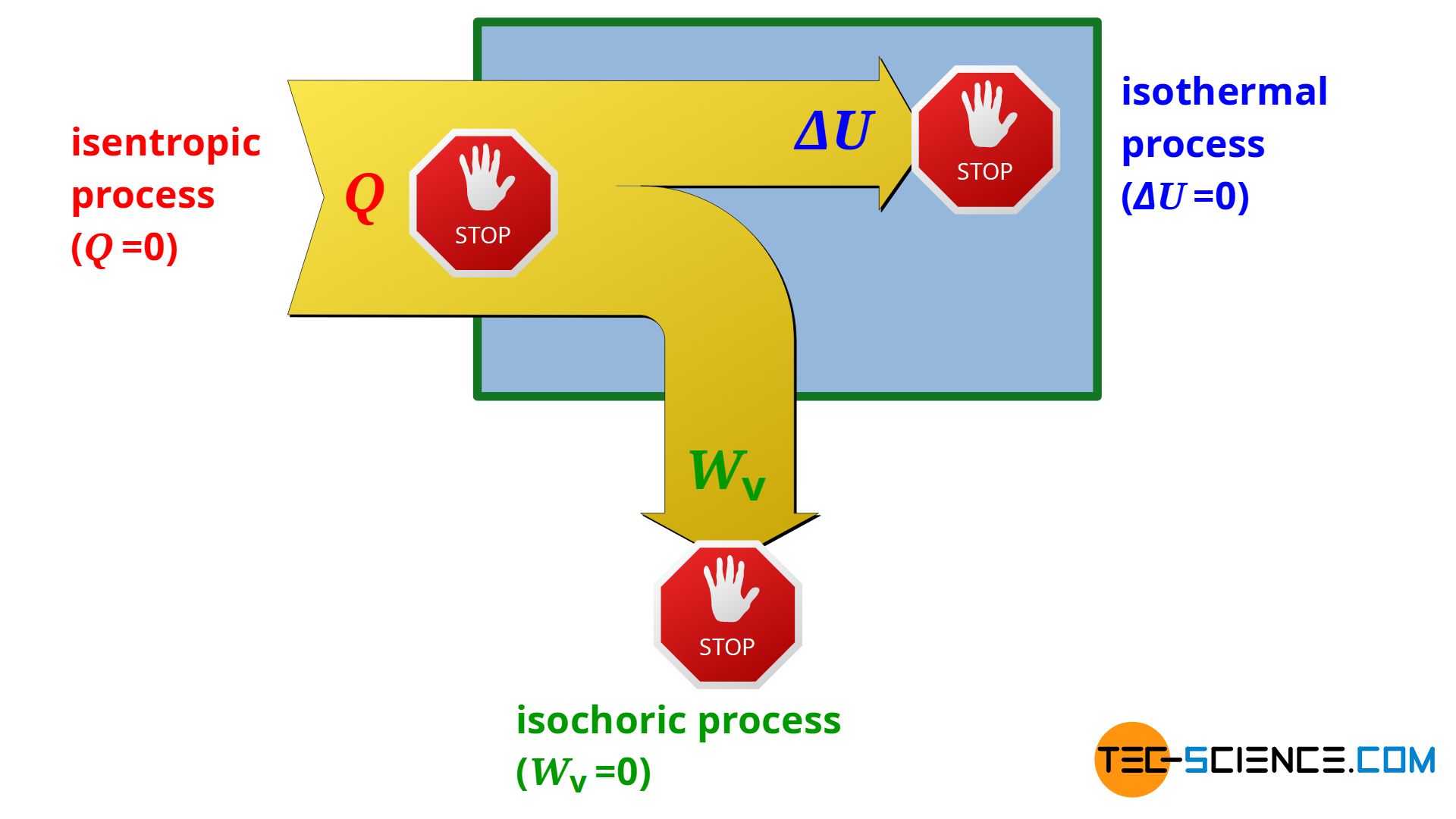
Adiabatic Process Animation #kineticschool #thermodynamicschemistry #thermodynamicprocesschapter:0:13 definition thermodynamic process1:33 types of thermodynamic processes1:53 isother. This simulation shows animations and calculates work for reversible and irreversible expansions and compressions (adiabatic or isothermal) of an ideal diatomic gas in a piston cylinder system. select either compression or expansion using the drop down menus, and compare two processes side by side. change the final pressure with the slider; the. The surprising result of this process shows up, if one takes a look at the first law of thermodynamics: w =0 q =0 = Δu = 0 (1) (1) w ⏟ = 0 q ⏟ = 0 = Δ u = 0. figure: adiabatic expansion of an ideal gas against a vacuum (final state) since it is an adiabatic system, by definition no heat is transferred (q=0). An adiabatic process is one in which no heat is gained or lost by the system. the first law of thermodynamics with q=0 shows that all the change in internal energy is in the form of work done. this puts a constraint on the heat engine process leading to the adiabatic condition shown below. this condition can be used to derive the expression for.

Adiabatic Process What Is Adiabatic Process Youtube The surprising result of this process shows up, if one takes a look at the first law of thermodynamics: w =0 q =0 = Δu = 0 (1) (1) w ⏟ = 0 q ⏟ = 0 = Δ u = 0. figure: adiabatic expansion of an ideal gas against a vacuum (final state) since it is an adiabatic system, by definition no heat is transferred (q=0). An adiabatic process is one in which no heat is gained or lost by the system. the first law of thermodynamics with q=0 shows that all the change in internal energy is in the form of work done. this puts a constraint on the heat engine process leading to the adiabatic condition shown below. this condition can be used to derive the expression for. δw = −pdv δ w = − p d v. the differential statement of the first law of thermodynamics is an equation of the inexact differentials of heat and work (because heat and work are process energies) to the exact differential of internal energy: du = δq δw d u = δ q δ w. because δq is equal to zero in an adiabatic process (if no heat. Because we are modeling the process as a quasi static adiabatic compression of an ideal gas, we have pvγ = constant and pv = nrt. the work needed can then be evaluated with w = ∫v2v1pdv. solution. for an adiabatic compression we have p2 = p1(v1 v2)γ, so after the compression, the pressure of the mixture is p2 = (1.00 × 105n m2)(240 × 10.

Comments are closed.