Acid Base Titration Lab Report Chem 1001 Pdf Lomoarcpsd 19803480 Ac

Acid Base Titration Lab Report Chem 1001 Pdf Lomo Chem 1001. acid base titrations laboratory report. name: rj hamdan date: 11 8 section: 432 ta: ben kies. a. goals purpose of experiment (2 points, ~50 words): the goal of this experiment is using the skills of precision to determine the equivalence point of various titration trials. View acid base titration lab report chem 1001.pdf from chem 1001 at carleton university. lomoarcpsd|19803480 acid base titration lab report chem 1001 general chemistry i (carleton university) studocu ai chat with pdf.

Acid Base Titrations Lab Report Pdf Chem 1001 Aci Chem 1210. spring 2019. experiment #10 11:part 1 acid base titration. abstract: the purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide, a strong base. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Indicator. references. acid base titrations are usually used to find the amount of a known acidic or basic substance through acid base reactions. the analyte (titrand) is the solution with an unknown molarity. the reagent (titrant) is the solution with a known molarity that will react with the analyte. Be able to determine the molar mass of a solid monoprotic acid from titration data; be able to calculate k a1 and k a2 for a polyprotic acid; by the end of this lab, students should be able to: design, develop and perform acid base titrations . prior knowledge: graphing; stoichiometry of acid base titrations in quantitative analysis (section 4.7.2).
Sample Lab Report On Acid Base Titration At Michelle Hamilton Blog Indicator. references. acid base titrations are usually used to find the amount of a known acidic or basic substance through acid base reactions. the analyte (titrand) is the solution with an unknown molarity. the reagent (titrant) is the solution with a known molarity that will react with the analyte. Be able to determine the molar mass of a solid monoprotic acid from titration data; be able to calculate k a1 and k a2 for a polyprotic acid; by the end of this lab, students should be able to: design, develop and perform acid base titrations . prior knowledge: graphing; stoichiometry of acid base titrations in quantitative analysis (section 4.7.2). In acid base. titration, the data recorded are named as paired values (volume of base added and the ph of. solution). our intention is to construct a graph (volume of base added vs the ph of the. These are acid base reactions, so you should be able to write the equation for the reaction that occurs with each titration. (acid base → salt water). in this experiment you will make solutions of “khp" (potassium hydrogen phthalate), a primary standard with molar mass 204.223 g mol. khp has one titratable h.

Lab Report Acid Base Pdf Titration Chemistry In acid base. titration, the data recorded are named as paired values (volume of base added and the ph of. solution). our intention is to construct a graph (volume of base added vs the ph of the. These are acid base reactions, so you should be able to write the equation for the reaction that occurs with each titration. (acid base → salt water). in this experiment you will make solutions of “khp" (potassium hydrogen phthalate), a primary standard with molar mass 204.223 g mol. khp has one titratable h.

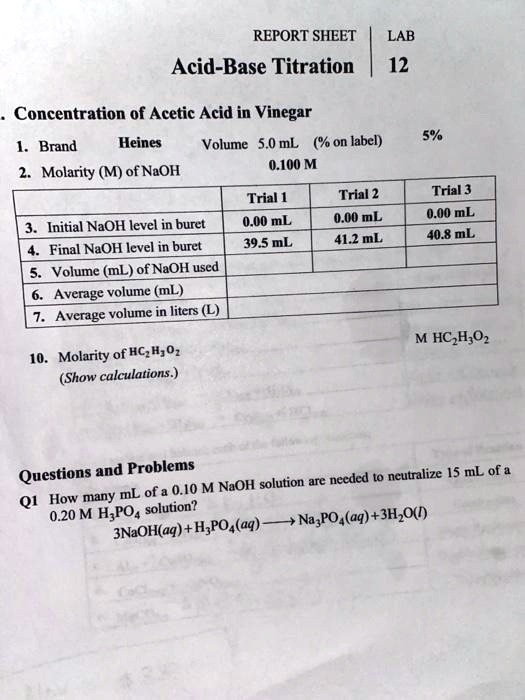
Acid Basetitrationslabreport11 7 17 Docx Chem 1001 Acid Base

Comments are closed.