Acid Base Extraction Flow Chart

Organic Chemistry Extraction Flow Chart An acid base extraction can be used to extract carboxylic acids from the organic layer into the aqueous layer. as was discussed in the previous section, naoh naoh can be used to convert a carboxylic acid into its more water soluble ionic carboxylate form. however, if the mixture contains a desired compound that can react with naoh naoh, a. Acid base extraction flow chart; references; experimental aims: the objective of this exercise is to separate a two component mixture using extraction techniques and then to identify the isolated components by determining their melting points. experimental learning objectives: at the end of this experiment you should be able to:.
Acid Base Extraction Flow Chart An acid base extraction is a type of liquid liquid extraction. it typically involves different solubility levels in water and an organic solvent. the organic solvent may be any carbon based liqiuid that does not dissolve very well in water; common ones are ether, ethyl acetate, or dichloromethane. acid base extraction is typically used to. Chem 344 extraction flowchart . crude . neutral. unknown mixture conjugate base of acid 2 rco na base (rnh 2) neutral (n) conjugate acid of base (rnh 3 cl ) crude ppt. of amine r nh 2 crude ppt. of acid rco 2 h rco 2 h acid rnh 2 base n neutral extract with 5% naoh organic phase aqueous phase extract with 10% hcl evap. org. phase aqueous. Acid base extractiona. id base extraction.1extraction involves dissolving a compound or compounds either (1) from a solid into a solvent or (2) from a solution. nto another solvent. a familiar example of the first case is making a cup of tea or coffee: the soluble flavor, odor chemicals, and caffeine are extracted from the solid tea leaves or. Just as washing your hands with soap and water can remove greasy impurities, washing an organic solvent, contaminated with a small amount of acid impurity, with an aqueous solution of base (naoh, na2co3) will remove that acid impurity by forming a water soluble salt. you will be doing both extraction and washing in today’s experiment.
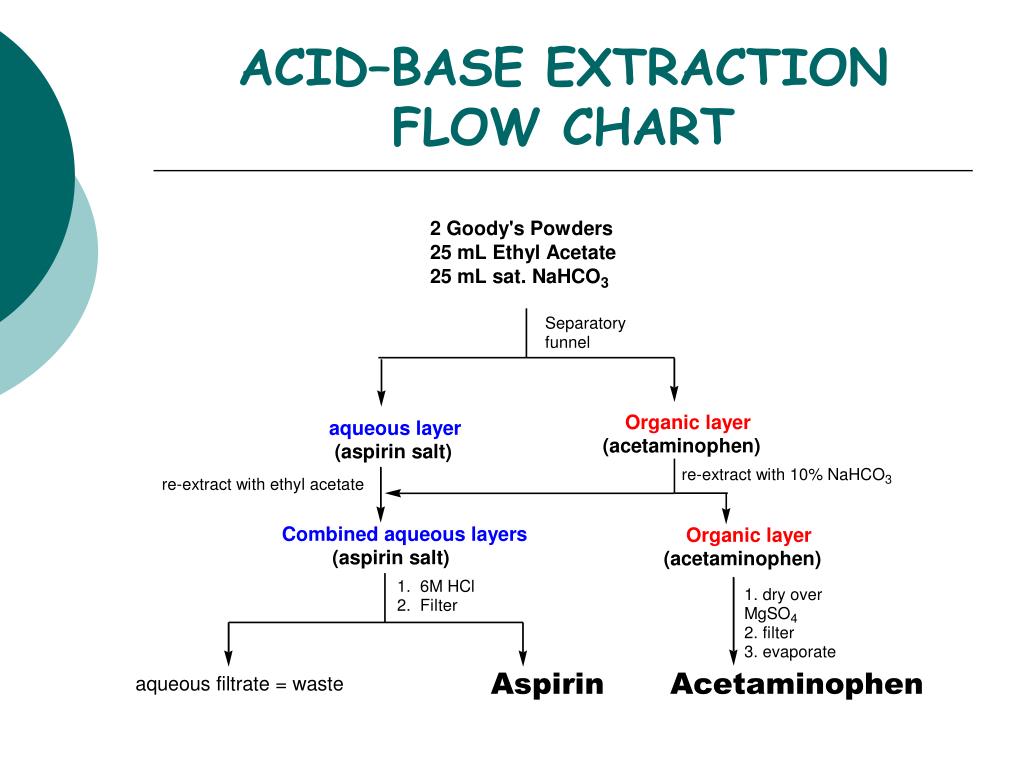
Acid Base Extraction Flow Chart Acid base extractiona. id base extraction.1extraction involves dissolving a compound or compounds either (1) from a solid into a solvent or (2) from a solution. nto another solvent. a familiar example of the first case is making a cup of tea or coffee: the soluble flavor, odor chemicals, and caffeine are extracted from the solid tea leaves or. Just as washing your hands with soap and water can remove greasy impurities, washing an organic solvent, contaminated with a small amount of acid impurity, with an aqueous solution of base (naoh, na2co3) will remove that acid impurity by forming a water soluble salt. you will be doing both extraction and washing in today’s experiment. An acid base extraction, this week's experiment, is a modification of the second case above; that is, it is a solvent solvent extraction. before looking at what makes it an acid base extraction, first consider solvent solvent extractions in general. a requirement of a solvent solvent extraction is that the two. Add ~10 ml 3m hcl to the sep funnel. 5. seal the funnel and shake the mixture for ~30 seconds. remove the funnel cap, and allow the layers to separate. 6. drain the two layers into two separate erlenmeyer flasks. take care not to leave any dcm (bottom) in your aqueous (top) layer. label the organic flask as ‘organic’.

Acid Base Extraction Flow Chart An acid base extraction, this week's experiment, is a modification of the second case above; that is, it is a solvent solvent extraction. before looking at what makes it an acid base extraction, first consider solvent solvent extractions in general. a requirement of a solvent solvent extraction is that the two. Add ~10 ml 3m hcl to the sep funnel. 5. seal the funnel and shake the mixture for ~30 seconds. remove the funnel cap, and allow the layers to separate. 6. drain the two layers into two separate erlenmeyer flasks. take care not to leave any dcm (bottom) in your aqueous (top) layer. label the organic flask as ‘organic’.

Comments are closed.