A Grignard Reaction

The Grignard Reaction Mechanism Chemistry Steps The grignard reaction (french: [ɡʁiɲaʁ]) is an organometallic chemical reaction in which, according to the classical definition, carbon alkyl, allyl, vinyl, or aryl magnesium halides (grignard reagent) are added to the carbonyl groups of either an aldehyde or ketone under anhydrous conditions. [1][2][3] this reaction is important for the. The grignard reaction is the addition of an organomagnesium halide (grignard reagent) to a ketone or aldehyde, to form a tertiary or secondary alcohol, respectively. the reaction with formaldehyde leads to a primary alcohol. grignard reagents are also used in the following important reactions: the addition of an excess of a grignard reagent to.
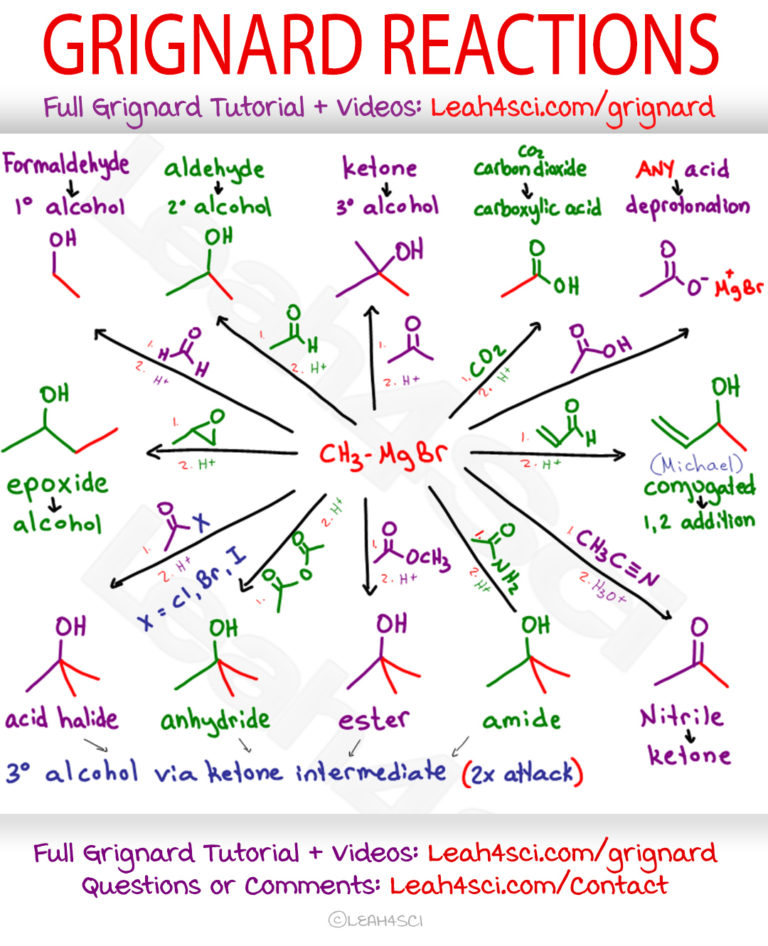
Grignard Reaction Mechanism Reagent And Cheat Sheet The reactions are essentially identical to the reaction with carbon dioxide all that differs is the nature of the organic product. in the first stage, the grignard reagent adds across the carbon oxygen double bond: dilute acid is then added to this to hydrolyse it. The grignard reaction uses an organometallic compound to make new carbon carbon bonds. simply put, the grignard reaction is a cornerstone process in organic chemistry that involves reacting grignard reagents (compounds containing a carbon magnesium bond) with a variety of other compounds to form a new carbon carbon bond. The grignard reaction, although useful, does have limitations. one major problem is that a grignard reagent can’t be prepared from an organohalide if other reactive functional groups are present in the same molecule. for example, a compound that is both an alkyl halide and a ketone can’t form a grignard reagent because it would react with. The grignard reaction is a prominent textbook process to form carbon–carbon bonds. (1) in this reaction, the so called grignard reagent, an organomagnesium species rmgx where r is an organic residue and x is a halogen (usually cl or br), promotes the addition of its organic residue to an electrophilic substrate.

Comments are closed.