5 1b Pv Work General Chemistry

5 1b Pv Work General Chemistry Youtube Chad expounds on the topic of pv work (pressure volume work) introduced in the last lesson. chad shows how it is equivalent to the definition of work presen. We can calculate the work done by the system for our example above. the gas has expanded from 9 to 10 liters. Δv = 10.0 mol – 9.0 mol = 1.0 mol. if the gas expanded against an external pressure of 5.0 atm: w = pΔv = 1.0 l x 5.0 l = 5.0 l⋅atm. the work done by the system has the unit, l⋅atm, and the units need to be in j or kj.

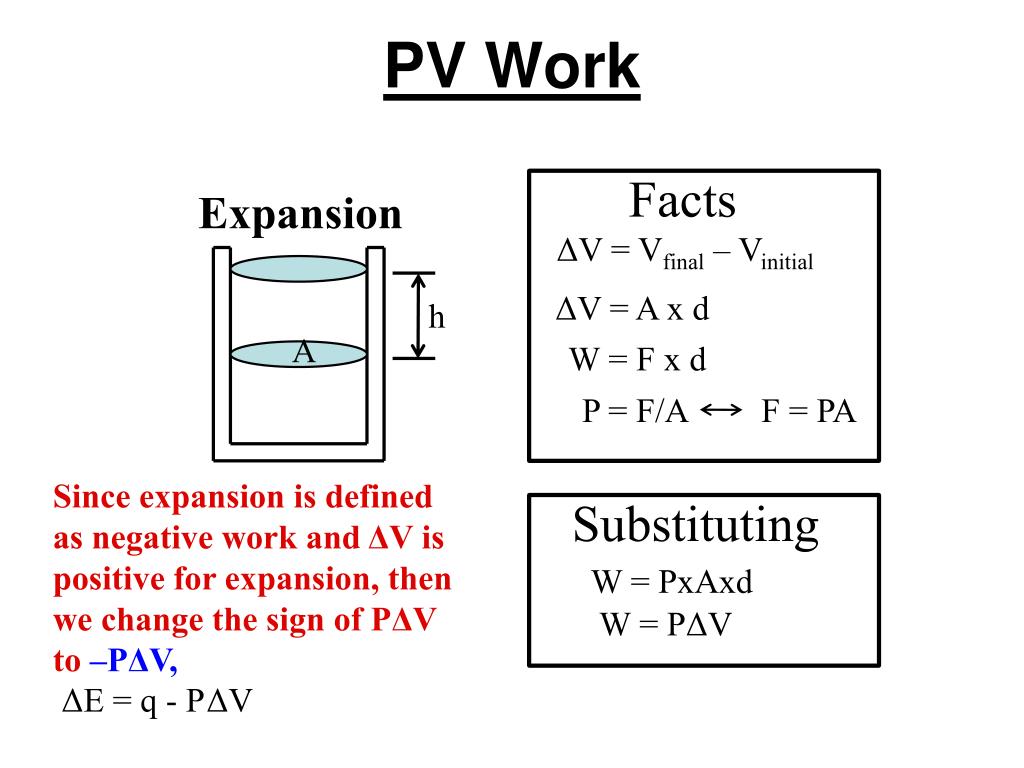
5 1b Ionic Compound Intro Science Chemistry Nomenclature Showme Chemistry lecture introducing pressure volume work (pv work). this is the type of work associated with a gas compression expansion. the equation for pv work. General chemistry complete study guide. 1.1 matter (26:39) matter quiz (7 questions) 1.2 scientific notation & significant figures (22:43) significant figures quiz (5 questions) 1.3 units and conversions (31:27) conversions and density quiz (20 questions) chapter 2 study guide. 2 – atoms, molecules, and ions 10 topics. The piston moves as the molecules of the gas rapidly equilibrate to the applied pressure such that the internal and external pressures are the same. the result of this motion is work: wvolume = ∫(f a) (ads) = ∫ pdv (19.2.1) this particular form of work is called pressure volume (pv) work and will play an important role in the development of. General chemistry: atoms first by john e. mcmurry and robert c. fay worked example 8.2 calculating e for a reaction continued problem 8.5 the reaction between hydrogen and oxygen to yield water vapor has h° = –484 kj: how much pv work is done, and what is the value of e in kilojoules for the reaction of 0.50 mol of h 2 with 0.25 mol of o.
The Concept Of Pv Work The piston moves as the molecules of the gas rapidly equilibrate to the applied pressure such that the internal and external pressures are the same. the result of this motion is work: wvolume = ∫(f a) (ads) = ∫ pdv (19.2.1) this particular form of work is called pressure volume (pv) work and will play an important role in the development of. General chemistry: atoms first by john e. mcmurry and robert c. fay worked example 8.2 calculating e for a reaction continued problem 8.5 the reaction between hydrogen and oxygen to yield water vapor has h° = –484 kj: how much pv work is done, and what is the value of e in kilojoules for the reaction of 0.50 mol of h 2 with 0.25 mol of o. Heat h2o(s) → h2o(l) Δh> 0. h2o(l) → h2o(s) heat Δh <0. in both cases, the magnitude of the enthalpy change is the same; only the sign is different. enthalpy is an extensive property (like mass). the magnitude of Δ h for a reaction is proportional to the amounts of the substances that react. Uci chem 1b general chemistry (winter 2013)lec 05. work depends on how you got there pv work 47:09 problem 50:22 equation: reversible.
Ppt Chapter 5 Powerpoint Presentation Free Download Id 4342222 Heat h2o(s) → h2o(l) Δh> 0. h2o(l) → h2o(s) heat Δh <0. in both cases, the magnitude of the enthalpy change is the same; only the sign is different. enthalpy is an extensive property (like mass). the magnitude of Δ h for a reaction is proportional to the amounts of the substances that react. Uci chem 1b general chemistry (winter 2013)lec 05. work depends on how you got there pv work 47:09 problem 50:22 equation: reversible.

Comments are closed.