4 Transition Elements Qns Topic Transition Elements Section A

4 Transition Elements Qns Topic Transition Elements Section A [1 μg = 1 x 10 6 g] [8] [nov 2004 (paper 3) (q4)] 29 (a) alloys are mixtures of two or more metals. transition elements are usually alloyed with one another to enhance their properties. for instance, titanium can be alloyed with iron and copper to form a strong, light weight and corrosion resistant material for aerospace and automobile. Properties of the transition elements. transition metals demonstrate a wide range of chemical behaviors. as can be seen from their reduction potentials (see appendix h), some transition metals are strong reducing agents, whereas others have very low reactivity.
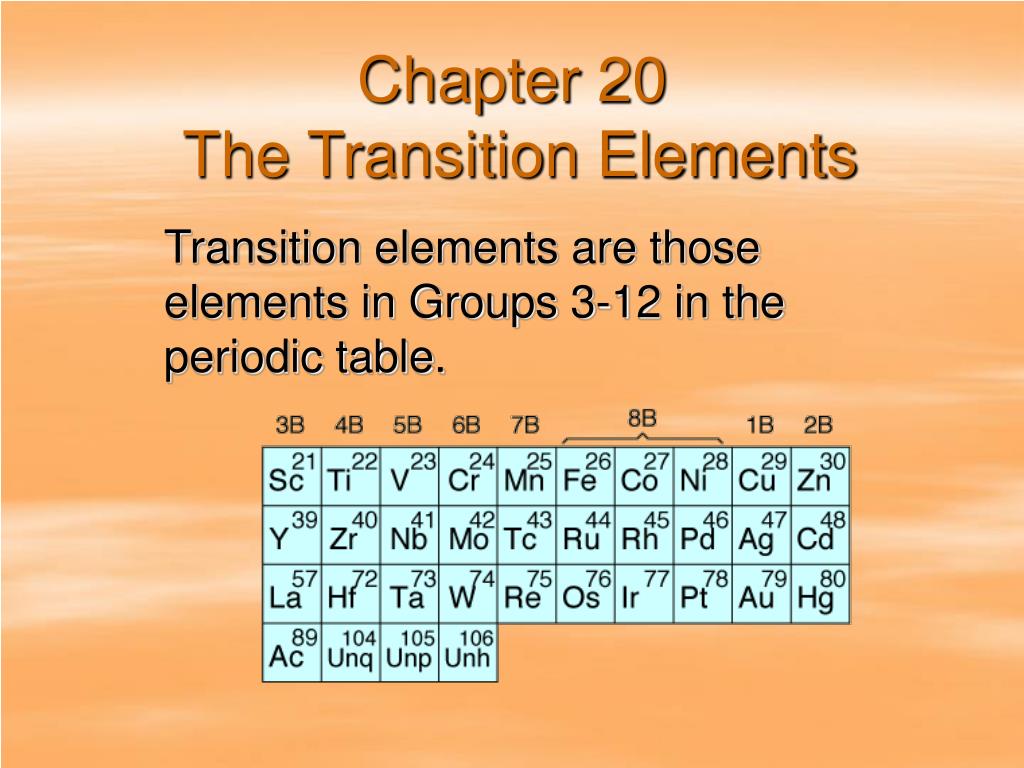
Ppt Chapter 20 The Transition Elements Powerpoint Presentation Free The visible light of a given energy level that is not absorbed produces a distinctly colored solution. figure 6.13.4 6.13. 4: transition metal compounds dissolved in water exhibit a wide variety of bright colors. from left to right are shown solutions of cobalt (ii) nitrate, potassium dichromate, potassium chromate, nickel (ii) chloride, copper. Iupac defines transition elements as an element having a d subshell that is partially filled with electrons, or an element that has the ability to form stable cations with an incompletely filled d orbital. in general, any element which corresponds to the d block of the modern periodic table (which consists of groups 3 12) is considered to be a. Transition elements are found in the d block of the periodic table. transition elements form one or more stable ions with incomplete d orbitals. the d subshell can hold up to 10 electrons. because their ions do not have an incomplete d subshell. scandium forms one stable ion, sc3 , which has an empty d sub shell. The 3d block includes the first series of transition elements and make up part of period 4 of the periodic table a transition element is a metal whose atoms or at least one ion has a partially filled d subshell. scandium and zinc are not true transition metals but are still 3d block elements. typical properties of transition elements:.

Ppt Topic 4 The Periodic Table And Some Atomic Properties Transition elements are found in the d block of the periodic table. transition elements form one or more stable ions with incomplete d orbitals. the d subshell can hold up to 10 electrons. because their ions do not have an incomplete d subshell. scandium forms one stable ion, sc3 , which has an empty d sub shell. The 3d block includes the first series of transition elements and make up part of period 4 of the periodic table a transition element is a metal whose atoms or at least one ion has a partially filled d subshell. scandium and zinc are not true transition metals but are still 3d block elements. typical properties of transition elements:. When distributing the activity section to the learners either as a printed copy or as a word file you will need to remove the teacher instructions section. the activity. this lesson element is a teaching and learning resource containing 10 multiple choice questions (mcqs) on the theme of transition elements and qualitative analysis. List of transition metal elements. using the iupac definition, there are 40 transition metals. they are: atomic numbers 21 (scandium) to 30 (zinc) atomic numbers 39 (yttrium) to 48 (cadmium) atomic numbers 71 (lutetium) to 80 (mercury) atomic numbers 103 (lawrencium) to 112 (copernicium) the full list is: scandium.

Comments are closed.