Floating Egg In Salt Water Experiment

How To Make An Egg Float Kitchen Science For Kids Pour 1 ½ cups of water into your large container. add ½ cup of salt to the large container and stir to dissolve some of the salt (it will not all dissolve yet). add one more cup of water to the large container (making 2 ½ cups total) and stir to dissolve the rest of the salt. the salt should be completely dissolved before you go on to the. Add one half cup of salt to the large container and stir to dissolve some of the salt (it will not all dissolve yet). add one more cup of water to the large container (making two and one half cups.
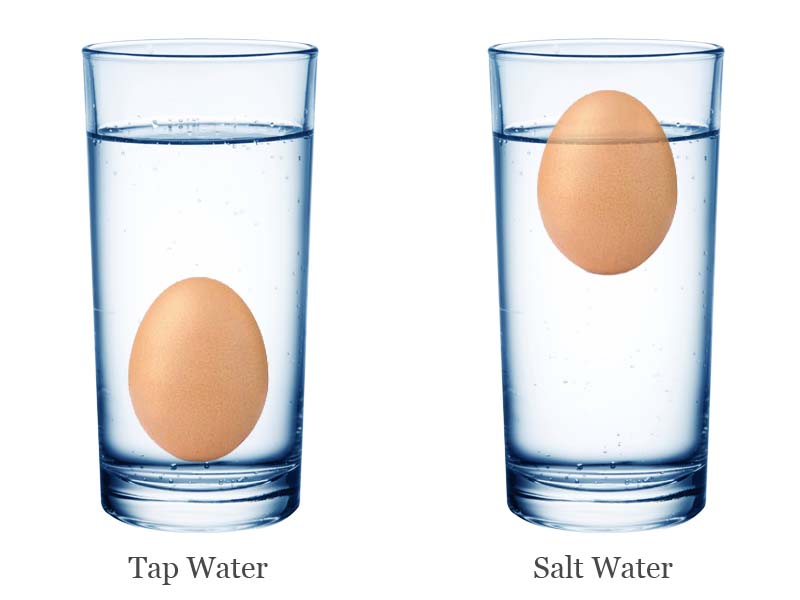
Floating Eggs In Salt Water Fun Experiment Science4fun Floating egg experiment set up. step 1: start by filling one glass about 2 3 of the way full with water. ask the kids what will happen if you carefully drop an egg into the glass of water. now go ahead and do it! step 2: in the other glass, fill to the same height with water. now stir in 3 tablespoons of salt. mix well to dissolve the salt!. The egg will sink in regular tap water because the density of the egg is greater than the density of water. the egg’s density is only slightly higher than water at 1.03 g ml, but that is enough to make the egg sink. when you add salt to the water, you are increasing the density of the water by adding more mass (or stuff) in the given volume. Fresh water first and then salt water. i always start demonstrating the egg sinking in the fresh water first. next, you will show how adding salt to the fresh water will change its density. thus, the egg will now float in the denser salt water. in the egg floating experiment, i use 1 tablespoon of salt per 1 2 cup of water. Take five eggs out of the refrigerator, use a permanent marker to label them 1 5, and allow them to warm to room temperature. make a stock solution of 1 cup of salt dissolved in 5 cups of water, as follows: pour 3 cups of water into your large container. add 1 cup of salt. stir to dissolve some of the salt.

Salt Water Floating Egg Experiment Fresh water first and then salt water. i always start demonstrating the egg sinking in the fresh water first. next, you will show how adding salt to the fresh water will change its density. thus, the egg will now float in the denser salt water. in the egg floating experiment, i use 1 tablespoon of salt per 1 2 cup of water. Take five eggs out of the refrigerator, use a permanent marker to label them 1 5, and allow them to warm to room temperature. make a stock solution of 1 cup of salt dissolved in 5 cups of water, as follows: pour 3 cups of water into your large container. add 1 cup of salt. stir to dissolve some of the salt. Instructions: 1. pour water into the glass until it is about half full. 2. place an egg in the glass of water and see if it sinks or floats (it should sink). 2. stir in lots of salt. start with 1 tablespoon and stir it until the salt dissolves. keep adding more salt until the egg floats. Learn about density and floating objects with this easy and fun activity. compare how an egg behaves in different liquids and find out which one makes it float.

Salt Water Floating Egg Experiment Instructions: 1. pour water into the glass until it is about half full. 2. place an egg in the glass of water and see if it sinks or floats (it should sink). 2. stir in lots of salt. start with 1 tablespoon and stir it until the salt dissolves. keep adding more salt until the egg floats. Learn about density and floating objects with this easy and fun activity. compare how an egg behaves in different liquids and find out which one makes it float.
Egg Float In Salt Water Experiment

Comments are closed.