Chapter 16_lect 1_acid Base Equilibria Arrhenius Acid Base

Arrhenius Acids And Bases Chapter 16: acid–base equilibria chapter 16 acid base equilibria learning standards & objectives; chapter 16 acid base equilibria list the general properties that characterize acidic and basic solutions and i can: ap16 1,2 01 identify the ions responsible for these properties. ap16 1,2 02 define the terms bronsted lowry acid and base, and. Terms in this set (27) arrhenius acids and bases. acid produces h in water. base produces oh in water. neutralization reaction between an acid and a base producing a salt and water. bronsted lowry acids and bases. acid donates an h to another substance. base accepts an h from another substance.
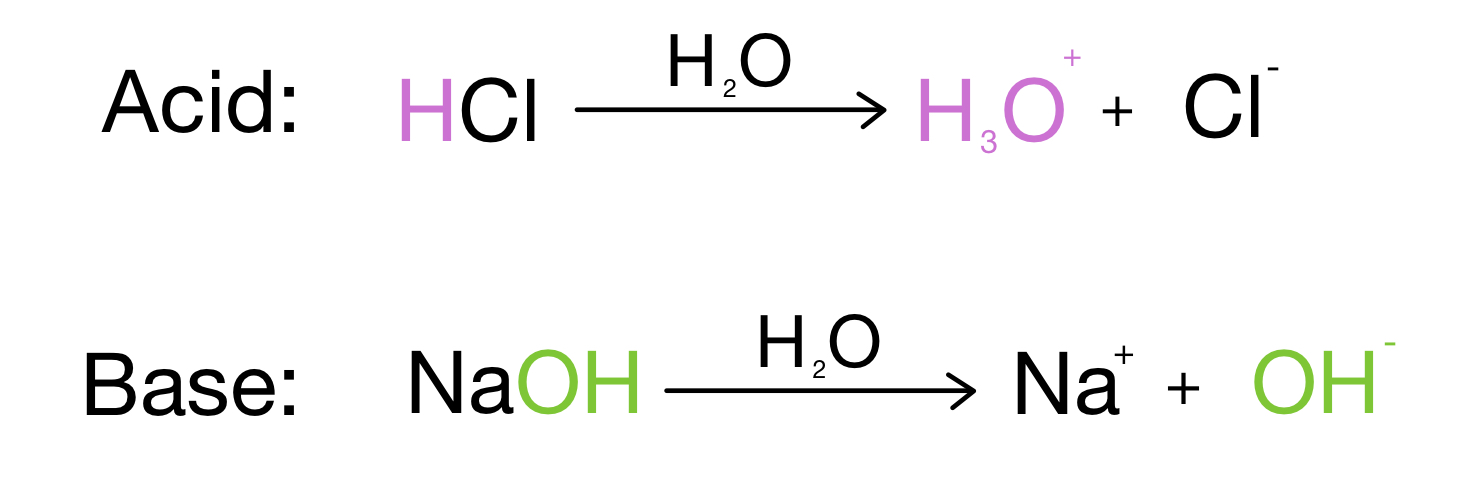
Arrhenius Acids And Bases вђ Definition Examples Expii Chapter 16 acid base equilibria. arrhenius concept of acids and bases: click the card to flip 👆. an acid is a substance that, when dissolved in water, increases the concentration of h^ ions. a base is a substance that, when dissolved in water, increase the concentration of oh^ ions. click the card to flip 👆. 1 61. Study with quizlet and memorize flashcards containing terms like arrhenius acids are compounds that increase the concentration in water, and arrhenius bases are compounds that increase the ion concentration in water, bronsted lowry acid, bronsted lowry base and more. Bh. (aq) 16.2 brønsted‐lowry acids & bases. ‐ the h ion in water ‐‐ h o . 3 is called the hydronium ion and is what truly happens when h is in water ‐‐ since all aqueous solution are in water we will use h3o & h interchangeably ‐ conjugate acid‐base pairs ‐‐ conjugate acid: the acid that is created after the brønsted. 1 chapter 16 acid base equilibria arrhenius definition produce hydrogen ions in aqueous solution. produce hydroxide ions when dissolved in water. limits to aqueous solutions. only one kind of base. nh 3 could not be an arrhenius base. bronsted lowry definitions.

Comments are closed.