Arrhenius Model Of Acids And Bases

Arrhenius Concept Of Acids And Bases Arrhenius Theory Youtube The reaction between an arrhenius acid and an arrhenius base is called neutralization and results in the formation of water and a salt. 7.1: arrhenius acids and bases is shared under a cc by nc sa 4.0 license and was authored, remixed, and or curated by libretexts. an arrhenius acid is a compound that increases the h ion concentration in. Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield electrically charged atoms or molecules, called ions, one of which is a hydrogen ion (h ), and that bases ionize in water to yield hydroxide ions (oh −). it is now known that the hydrogen ion.
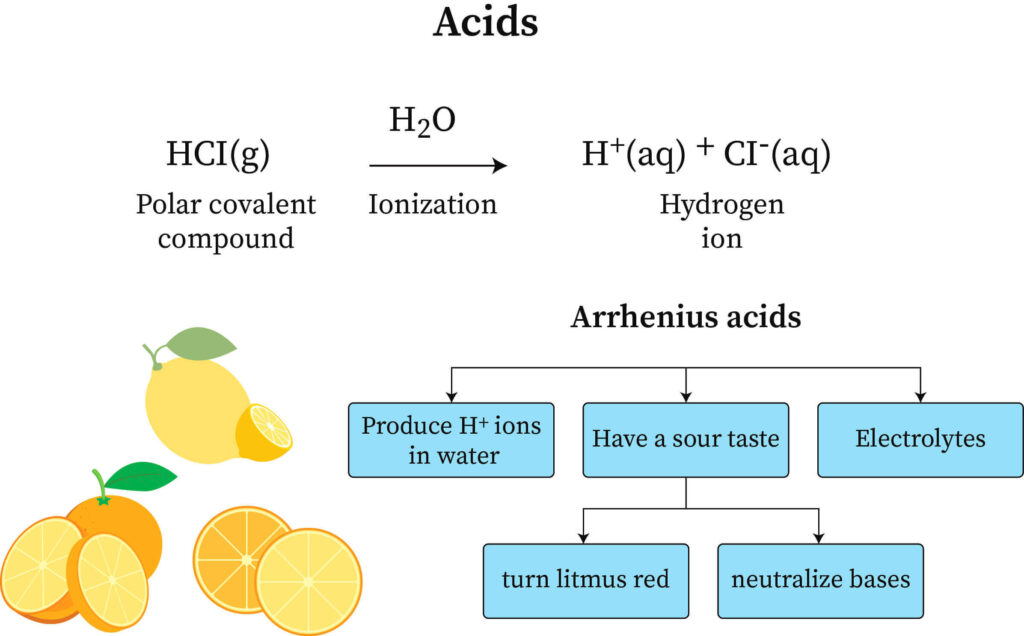
Arrhenius Equation Definition Examples And Theory Arrhenius acids and bases are the first types of acids and bases most students learn about in chemistry class. partly this is because arrhenius acid base theory is the first modern explanation of acids and bases based on molecules and ions. svante arrhenius’s hydrogen theory of acids in bases in 1884 earned him the nobel prize in chemistry in. 6.1: arrhenius model. the arrhenius acid base concept defines acids and bases in terms of how they affect the amount of hydronium ions, h 3o , (and by extension hydroxide ions, oh −) in aqueous solutions. simply, in the arrhenius definition an acid is a substance that increases the concentration of hydronium ions when it is dissolved in water. Arrhenius theory of acid and base. according to arrhenius theory, acid is a substance that gives h ions on dissolving in the aqueous solution. it increases the concentration of h ions in the solution. the base is a substance that ionises oh – ion by dissolving in the aqueous solution. the concentration of oh ions is high in the solution. The swedish chemist svante arrhenius developed the first chemical definitions of acids and bases in the late 1800s. arrhenius defined an acid as a compound that increases the concentration of hydrogen ion (h ) in aqueous solution. many acids are simple compounds that release a hydrogen cation into solution when they dissolve.
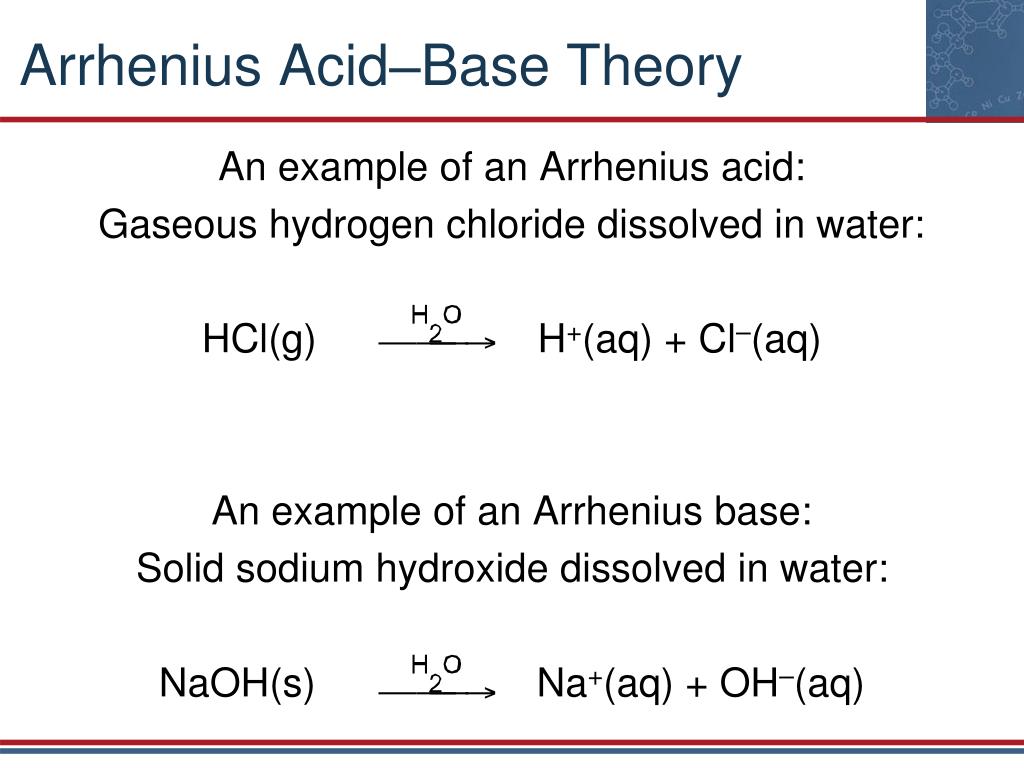
Ppt Chapter 17 Acidвђ Base Proton Transfer Reactions Powerpoint Arrhenius theory of acid and base. according to arrhenius theory, acid is a substance that gives h ions on dissolving in the aqueous solution. it increases the concentration of h ions in the solution. the base is a substance that ionises oh – ion by dissolving in the aqueous solution. the concentration of oh ions is high in the solution. The swedish chemist svante arrhenius developed the first chemical definitions of acids and bases in the late 1800s. arrhenius defined an acid as a compound that increases the concentration of hydrogen ion (h ) in aqueous solution. many acids are simple compounds that release a hydrogen cation into solution when they dissolve. If you're seeing this message, it means we're having trouble loading external resources on our website. if you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Limitations. arrhenius theory defines acids and bases in aqueous solutions. swedish scientist svante arrhenius introduced the theory in 1887. according to this theory, an acid is a substance that gives off hydrogen ions (h ) in an aqueous solution, and a base produces hydroxide ions (oh –) in an aqueous solution.

Arrhenius Model Of Acids And Bases If you're seeing this message, it means we're having trouble loading external resources on our website. if you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Limitations. arrhenius theory defines acids and bases in aqueous solutions. swedish scientist svante arrhenius introduced the theory in 1887. according to this theory, an acid is a substance that gives off hydrogen ions (h ) in an aqueous solution, and a base produces hydroxide ions (oh –) in an aqueous solution.

Arrhenius Acids And Bases

Comments are closed.